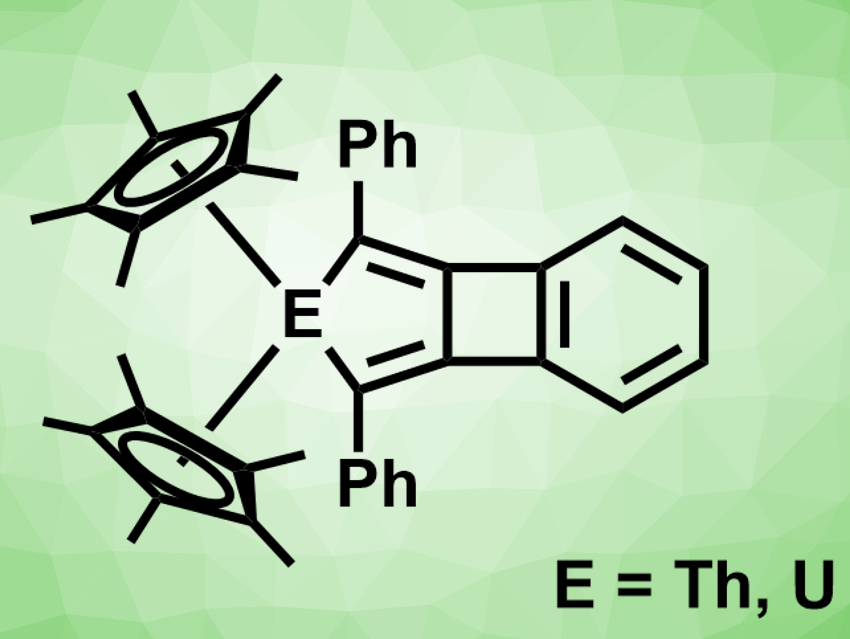
Biphenylene consists of two benzene rings, connected to form a four-membered ring between them. There are biphenylene analogues in which one benzene ring is replaced by a five-ring containing either a sulfur atom or an SO2 group. In contrast, no metallabiphenylenes had been reported so far.
David E. Morris, Jaqueline L. Kiplinger, Ping Yang, Los Alamos National Laboratory, NM, USA, Laura Gagliardi, University of Minnesota, Minneapolis, USA, and colleagues have synthesized the first 2-metallabiphenylene compounds (pictured). The team reduced the cyclopentadienyl complexes (C5Me5)2ECl2 (E = Th,U) with KC8 in the presence of 1,2-bis(phenylethynyl)benzene in toluene at 50 °C. They obtained the desired products in yields of 58 % for the thorium compound and 62 % for the uranium compound.
The products were characterized using X-ray diffraction (XRD) and NMR spectroscopy, and their electronic structure was investigated using density functional theory (DFT) calculations. The researchers found that both compounds have nearly planar tricyclic systems. The molecules combine an antiaromatic cyclobutadiene ring and an aromatic benzene ring that follow the Hückel rule. Due to their energy levels, the 5f orbitals play a larger role in the uranium compound than in the thorium compound. As a result, the uranium–metallabiphenylene bonding interaction has a substantial covalent character.
- Actinide 2-metallabiphenylenes that satisfy Hückel’s rule,
Justin K. Pagano, Jing Xie, Karla A. Erickson, Stephen K. Cope, Brian L. Scott, Ruilian Wu, Rory Waterman, David E. Morris, Ping Yang, Laura Gagliardi, Jaqueline L. Kiplinger,
Nature 2020.
https://doi.org/10.1038/s41586-020-2004-7