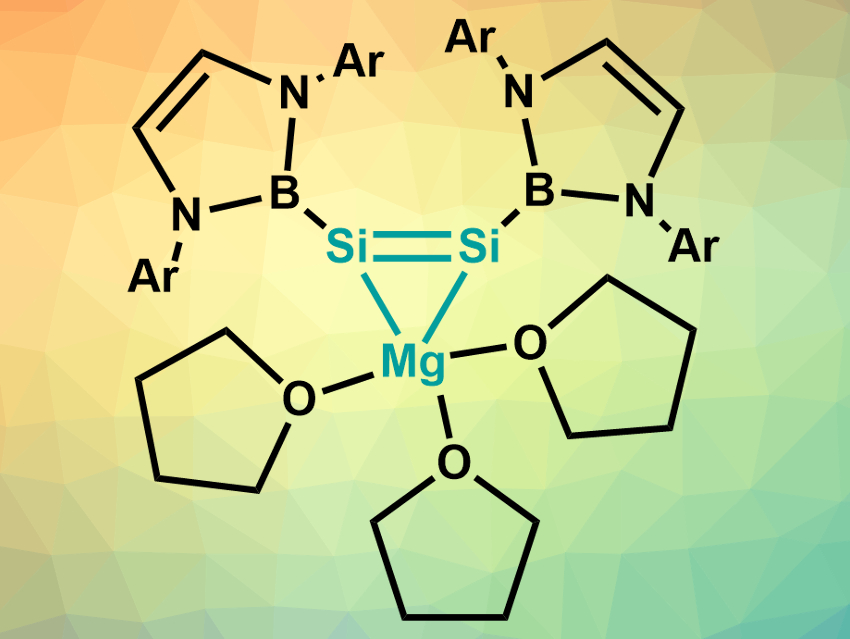
Silicon acts very differently from the lighter carbon in its multiply bonded compounds. Molecules with silicon–silicon double or triple bonds are much rarer than the corresponding carbon compounds and generally require bulky stabilizing groups. There are few examples of multiply bonded anionic silicon species, for example.
Chunming Cui, Nankai University, Tianjin, China, and colleagues have synthesized the first 1-magnesium-2,3-disilacyclopropene (pictured)—a magnesium complex of a dianionic disilyne. The team reduced the boryl ligand derivative (HCArN)2BSiBr3 (Ar = 2,6-iPr2C6H3) with activated magnesium in tetrahydrofuran (THF) to obtain the desired compound. The product was isolated as a yellow powder with a yield of 79 %.
The compound was characterized using 1H, 13C, and 29Si NMR spectroscopy, UV–Vis spectroscopy, and X-ray diffraction. The team found that it contains an unprecedented Mg–Si–Si three-membered ring. The silicon atoms have a nearly planar coordination. The structure and the results of density functional theory (DFT) calculations point to the existence of a Si═Si double bond. According to the team, the product could be useful for the synthesis of other new silicon compounds.
- Isolation of a 1-Magnesium-2,3-disilacyclopropene and a Related Bis(disilenide),
Miao Tian, Jianying Zhang, Hao Yang, Chunming Cui,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c00519