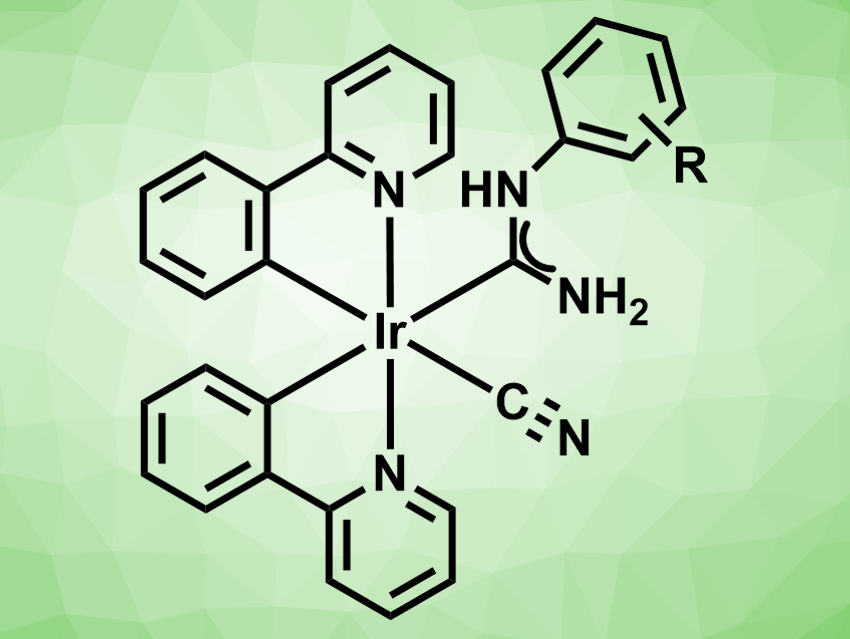
Mercury is a toxic heavy metal. Methods for its detection are, thus, important. Phosphorescent iridium(III) complexes, for example, can be used to detect heavy metals. Existing methods for this are based on either the coordination of mercury or the mercury-mediated dissociation of other ligands in the complex.
Konstantin V. Luzyanin, Saint Petersburg State University, Russia, and University of Liverpool, UK, Mikhail A. Kinzhalov, Saint Petersburg State University, and colleagues have synthesized bis(cyclometalated) iridium(III) species with ancillary isocyanides and acyclic diaminocarbene ligands (pictured), which can detect mercury by selectively reacting with the metal. The complexes were prepared from [(ppy)2Ir(μ-Cl)]2 (ppy = 2-phenylpyridine) and isocyanides of the type CNC6H4-4-X (X = F, Cl, BR, I).
The complexes show a blue-green phosphorescence at room temperature. When mercury(II) compounds were added to the phosphorescent solution, the phosphorescence intensity dropped significantly, particularly for the chloro derivative. This effect is selective, other heavy metal ions such as Cd2+ or Pb2+ do not reduce the phosphorescence intensity. The team proposes that the mercury binds to the isocyanide ligands of two of the iridium complexes and forms a bridged dimer with changed optical properties.
- Phosphorescent Iridium(III) Complexes with Acyclic Diaminocarbene Ligands as Chemosensors for Mercury,
Anzhelika A. Eremina, Mikhail A. Kinzhalov, Evgene A. Katlenok, Andrey S. Smirnov, Elena V. Andrusenko, Evgeny A. Pidko, Vitalii V. Suslonov, Konstantin V. Luzyanin,
Inorg. Chem. 2020.
https://doi.org/10.1021/acs.inorgchem.9b02833