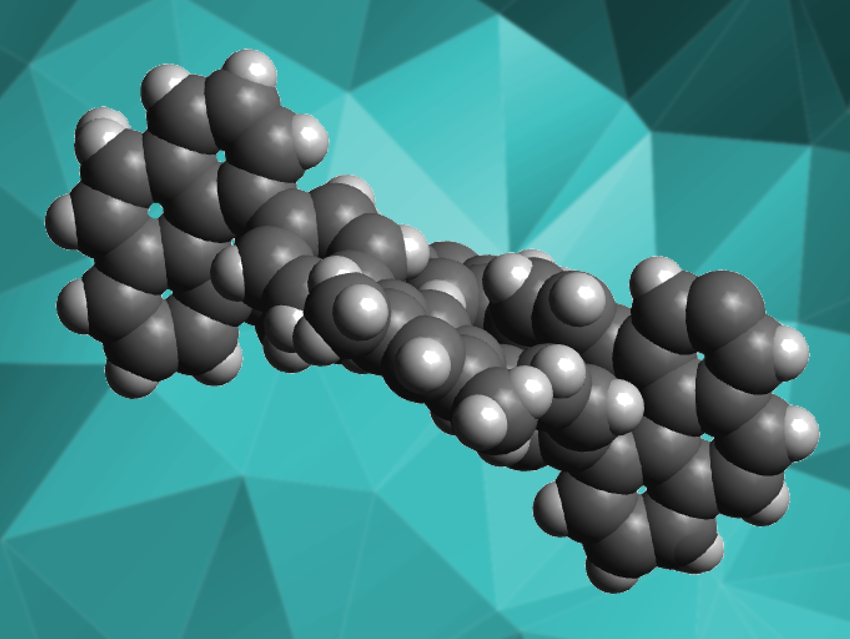
Planar aromatic molecules often aggregate due to π-stacking. In nonplanar aromatic molecules, this effect is reduced, which changes the compounds’ properties and can make them more useful, e.g., for optoelectronic applications or host-guest chemistry. The synthesis of such nonplanar aromatics often starts with an aromatic building block that is inherently twisted and/or three-dimensional, such as triptycene or 1,1′-binaphthalene.
Kenichiro Itami, Nagoya University, Japan, and colleagues have used a new 3D building block, 4,5-diarylphenanthrene, to make a series of highly twisted aromatic macrocycles (example pictured). This building block is twisted due to the close proximity of the two aryl substituents on the three-ring phenanthrene. The team started from the boronic acid pinacol (BPin) ester derivative of 4,5-diarylphenanthrene and used a quadruple Suzuki–Miyaura coupling to connect it to dibromo-functionalized aromatic linkers. The macrocycle product contains two equivalents of both the 4,5-diarylphenanthrene ends and the aromatic linkers. The team used a variety of aromatic linkers, from simple 1,3-dibromobenzene to terphenyls to tetraphenylethylenes.
The desired products were obtained in yields of 5–33 %. They have a twist angle close to 90°. The macrocycles are axially chiral due to their twisted nature and show slow racemization, i.e., they have a high conformational stability. The products have interesting properties such as aggregation-induced emission (AIE) or solvatofluorochromism, i.e., different optical properties in different solvents.
- Synthesis of Highly Twisted, Nonplanar Aromatic Macrocycles Enabled by an Axially Chiral 4,5-Diphenylphenanthrene Building Block,
Yuanming Li, Akiko Yagi, Kenichiro Itami,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.9b13549