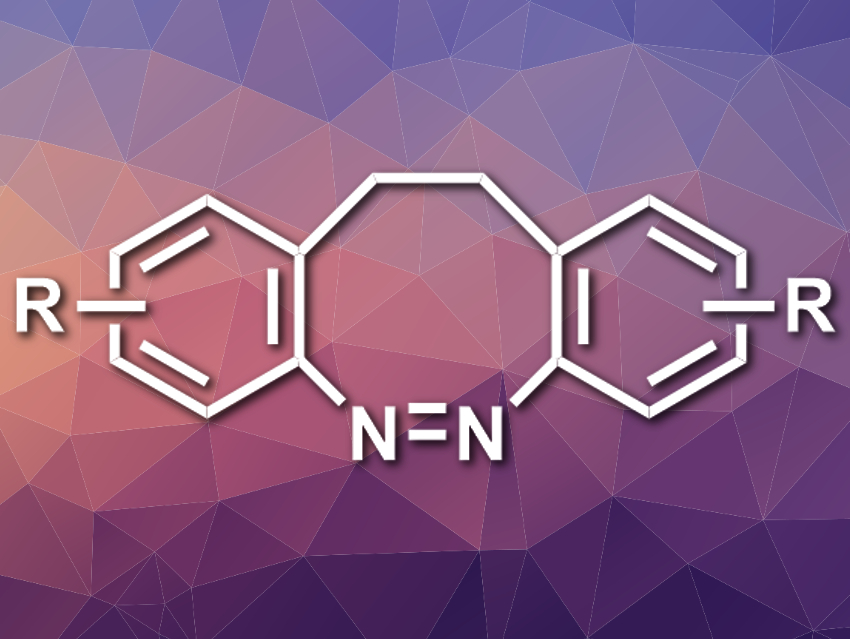
Diazocines are the ethylene-bridged derivatives of azobenzene (pictured). These molecules could be useful as molecular switches that can be isomerized using light. Their stability behaves opposite to the “normal”, linear azobenzene: Their (Z)-isomers are thermodynamically more stable than the (E)-isomers. Diazocines, however, are challenging to synthesize. Existing methods give low yields and/or have limited substrate scopes.
Anne Staubitz, University of Kiel and University of Bremen, both Germany, and colleagues have developed a new synthetic approach to diazocines. The method is based on inserting the diazo group between the two tethered benzene rings by consecutive C–N cross-coupling reactions. The team first prepared an ethylene-bridged precursor with two arene rings from two equivalents of a 2-bromobenzyl bromide derivative. Quenching the reaction with I2 gave an iodide-functionalized product. This product was reacted with di-tert-butyl hydrazodicarboxylate (DBADH2) in a copper-catalyzed cascade C–N coupling to give the desired closed ring system, which was then oxidized to the final product.
This three-step process is simple to execute and the desired products are obtained in moderate to excellent yields. The reaction tolerates a variety of substituents on the aryl rings, both electron-withdrawing and electron-donating. Heterocycles (thiophene or pyridine) can also be used in place of benzene derivatives.
- Cross-Coupling Strategy for the Synthesis of Diazocines,
Shuo Li, Nadi Eleya, Anne Staubitz,
Organic Letters 2020.
https://doi.org/10.1021/acs.orglett.0c00122