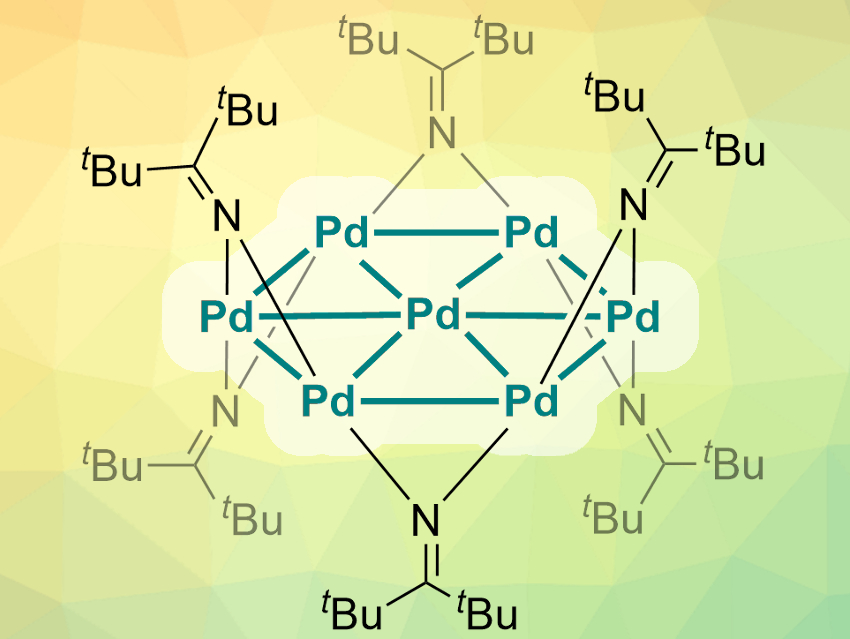
Metal nanoclusters can have interesting and often unexpected properties. Atomically precise nanoclusters (APNC) with palladium and platinum have mostly been stabilized by π-accepting ligands like carbon monoxide, phosphines, or isocyanides until now. APNCs of palladium are still very rare.
Trevor W. Hayton, University of California Santa Barbara, USA, Peter Hrobárik, Comenius University, Bratislava, Slovakia, and colleagues have discovered a particularly unique APNC with a core of seven palladium atoms (pictured). For the first time, ketimide ligands were used to stabilize an APNC. The team reduced a PdCl2(PhCN)2 precursor with the organolithium reagent Li(N=CtBu2) to obtain deep green crystals of the [Pd7(N=CtBu2)6] complex.
Single-crystal X-ray diffraction showed that the core forms a very rare hexagonal planar nanosheet with six outer Pd(I)-ions and one Pd(0)-atom at the center. The ketimide ligands are connected to this core in an alternating up/down fashion. The researchers used density-functional-theory (DFT) calculations to study the properties of the complex. They found that the metal core cluster is aromatic, with a ring current very similar to that of benzene.
The researchers point out that this is the first example of an all-metal nanocluster with this level of aromaticity and hexagonal-planar symmetry. This study may initiate research for other interesting group-10 nanoclusters with ketimide ligands.
- A Ketimide-Stabilized Palladium Nanocluster with a Hexagonal Aromatic Pd7 Core,
Andrew W. Cook, Peter Hrobárik, Peter L. Damon, Guang Wu, Trevor W. Hayton,
Inorg. Chem. 2020.
https://doi.org/10.1021/acs.inorgchem.9b03276