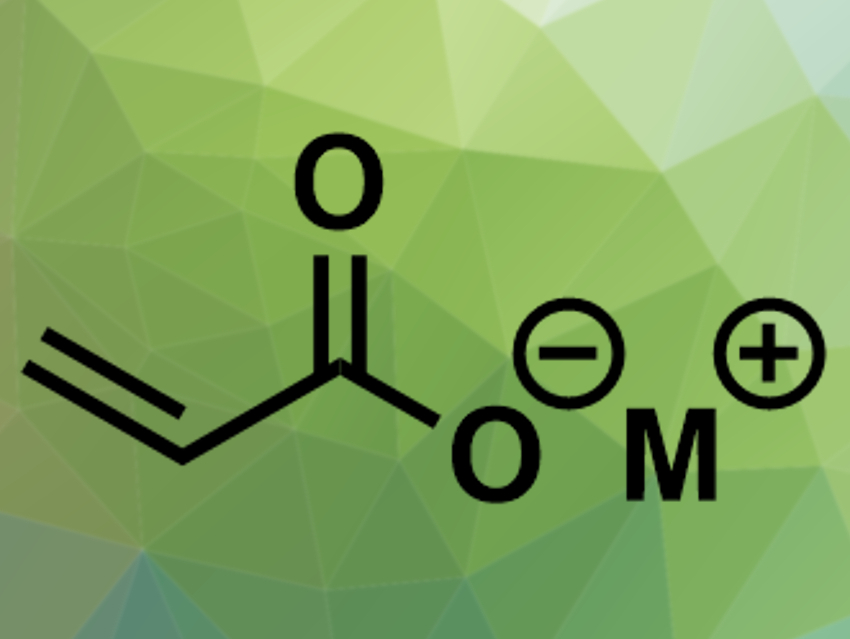
Acrylate salts are an important starting material for industrial chemical synthesis. One preparation method has recently regained interest: Zero-valent metal complexes, for example, with nickel or palladium, can catalyze the reaction of ethylene and carbon dioxide to acrylate salts. Converting this abundant greenhouse gas into a chemical building block could be useful to improve sustainability. The reaction is an oxidative cyclization with a metallalactone complex as the key intermediate.
Nobuharu Iwasawa, Tokyo Institute of Technology, Japan, and colleagues have studied the mechanism of the reaction of ethylene and carbon dioxide catalyzed by a phosphine-coordinated zero-valent ruthenium complex. The team’s detailed study of this reaction was possible because each key intermediate is isolable. The key intermediates in the catalytic cycle are an ethylene-coordinated Ru(0) complex, a ruthenalactone, and a hydrido acrylate complex.
The researchers first prepared four types of ethylene-coordinated Ru(0) complexes with different tetradentate phosphine ligands. They then investigated the electronic and steric influence of the ligand type on each step of the reaction. The team heated solutions of each complex under 1 atm of pressure of a 1:1 mixture of CO2 and ethylene. They monitored the reaction progress via 13C and 31P NMR spectroscopy.
The team experimentally verified two competing reaction mechanisms that had been predicted for the cleavage of the five-membered metallalactone. With the chosen ligands, the metallalactone intermediate can undergo either a β-hydride elimination or a direct deprotonation, depending on the strength of the base present. The team found that for their ruthenium complexes, the lactone intermediate can even be cleaved just by heating without extra reagents.
The researchers also found that the more electron-donating the ligands, the higher the reaction rate. They identified the rate-determining step, which involves the generation of a cationic ruthenium species when a carboxylate anion dissociates. This explains why ligands with more electron-donating properties accelerate the reaction. According to the researchers, these mechanistic insights could provide a boost for catalyst research to improve acrylate synthesis from carbon dioxide and ethylene.
- Mechanistic Investigations of the Ruthenium-Catalyzed Synthesis of Acrylate Salt from Ethylene and CO2,
Kohei Takahashi, Yo Hirataka, Tatsuyoshi Ito, Nobuharu Iwasawa,
Organometallics 2020.
https://doi.org/10.1021/acs.organomet.9b00659