Organoboronates are useful substrates for C–C bond-forming reactions. Diboronates, which have two boron atoms connected to the same carbon atom, are particularly reactive. They can, e.g., be deprotonated to give α-boryl carbanions, which can then serve as reactive intermediates in the preparation of a variety of organic compounds.
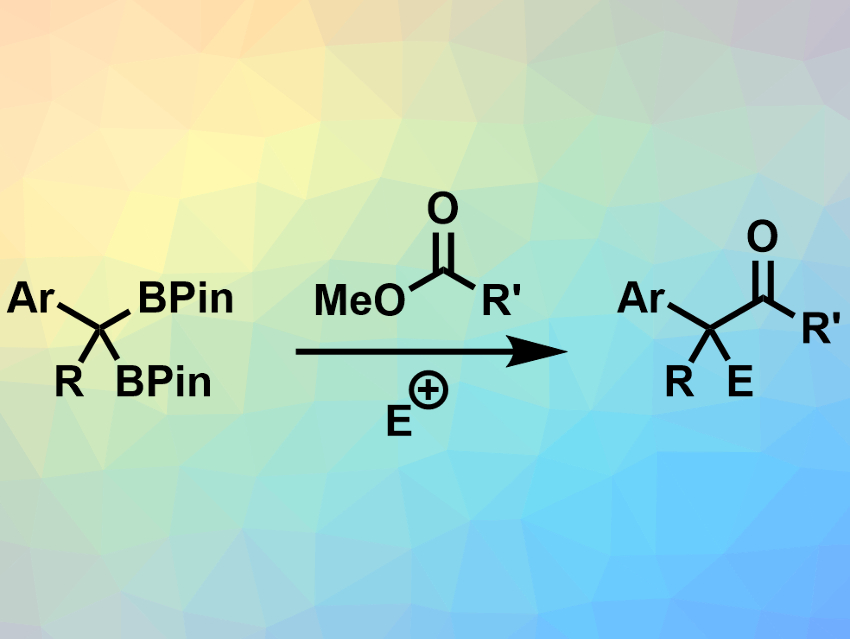
Boran Lee and Paul J. Chirik, Princeton University, NJ, USA, have used benzyldiboronates together with esters to synthesize functionalized ketones (example pictured). The team used bases such as lithium tert-butoxide (LiOtBu) to deprotonate and activate the benzyldiboronates in teterahydrofuran (THF) at room temperature. The activated substrate can react with a range of methyl esters to form the desired ketones. During this reaction, a boron enolate is formed as an intermediate. This enolate intermediate can be trapped with an electrophile (E+) to obtain α-functionalized ketones.
The reaction is easy to perform and has a broad substrate scope. It provides moderate to high yields of the desired ketones. When esters with asymmetric carbon atoms in the α-position were used, high enantiomeric excesses were retained in the final products. This shows that there is little racemization during the reaction.
- Ketone Synthesis from Benzyldiboronates and Esters: Leveraging α-Boryl Carbanions for Carbon–Carbon Bond Formation,
Boran Lee, Paul J. Chirik,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.9b11944