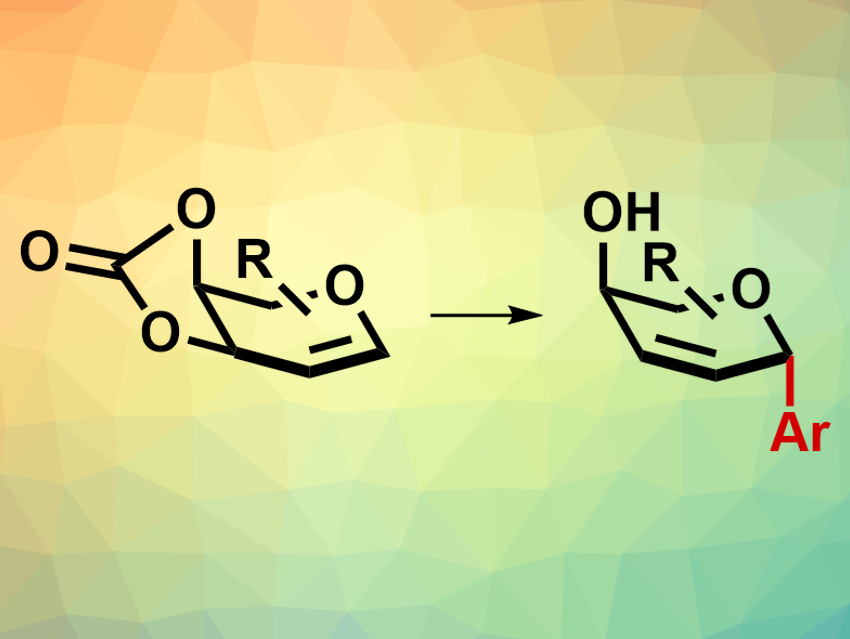
Carbohydrates and their derivatives are common natural products. C-glycosides, for example, are glycosides in which another carbon atom is connected to the anomeric carbon of a sugar. They are valuable building blocks for the synthesis of carbohydrate-based natural products and substances with potential pharmaceutical applications. Stereochemistry plays a fundamental role in determining the biological properties of these molecules. However, the stereoselective synthesis of C-glycosides remains challenging.
Hui Yao, Nianyu Huang, Kun Zou, China Three Gorges University, Yichang, and colleagues have developed a stereoselective synthesis route for C-aryl glycosides (pictured) from 3,4-O-carbonate glycals and aryl boronic acids. The reaction is catalyzed by the so-called White catalyst, a palladium(II)-acetate derived complex with the sulfoxide ligand 1,2-bis(phenylsulfinyl)ethane. The reactions were carried out in tetrahydrofuran (THF) at room temperature. With the White catalyst, moderate to high yields were achieved even when the reaction was exposed to air. Other Pd catalysts such as palladium acetate also gave good yields. However, due to their air instability, the reactions had to be carried out under inert conditions with these catalysts.
Many differently functionalized aryl boronic acids are tolerated by this reaction, including reactants with bulky functional groups in the para-position (e.g., isopropyl or tert-butyl) or with electron-withdrawing groups such as halogens or trifluoromethyl groups. The α-stereoselectivity of the reaction remained consistent with different aryl boronic acids and glycal precursors. The work provides an efficient and stereoselective path to C-glycosides using a commercially available catalyst which does not require air-free conditions or specialized equipment. This could make the products of this reaction more widely accessible.
- Open-Air Stereoselective Construction of C-Aryl Glycosides,
Mengnan Lai, Karwan Abdulmajed Othman, Hui Yao, Qiuyuan Wang, Yongkui Feng, Nianyu Huang, Mingguo Liu, Kun Zou,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.9b04665