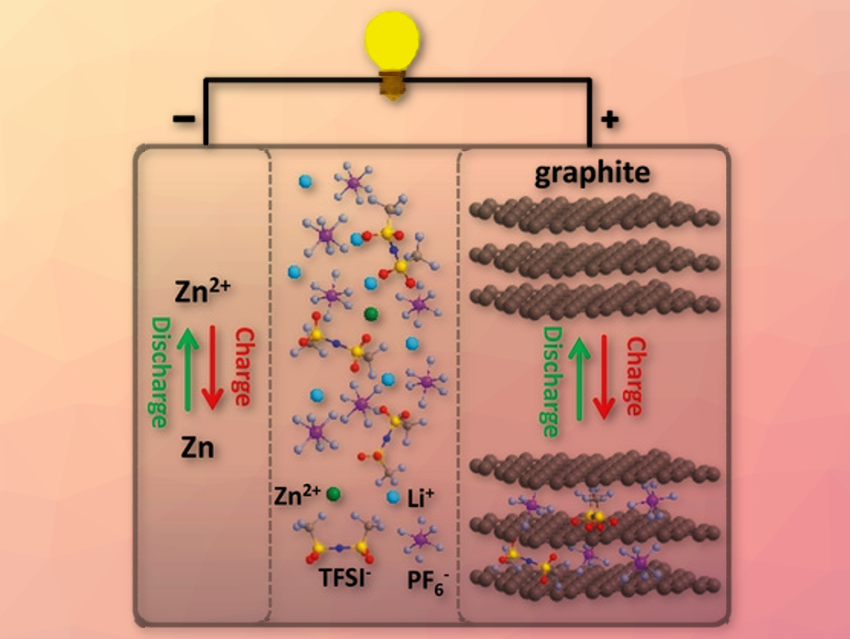
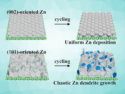
Zinc batteries have a high theoretical capacity. Moreover, Zn is a cheap and earth-abundant metal with low toxicity. However, Zn batteries suffer from dendrite formation and low voltages. Until now, there are few suitable electrolytes for Zn batteries with a sufficient electrochemical stability voltage. Moreover, Zn batteries show a poor cyclability until now.
Xinliang Feng, Technische Universität Dresden, Germany, and colleagues have developed a Zn battery overcoming some challenges mentioned above. It uses Zn nanosheets deposited on carbon cloth as an anode and graphite as a cathode. The electrolyte contains Zinc bis(trifluoromethanesulfonyl) imide (Zn(TFSI)2) dissolved in ethyl methylcarbonate solvent. As a crucial step, the team added LiPF6 in a distinct molar ratio to Zn(TFSI)2, creating a hybrid electrolyte.
Apart from in-depth analysis of the battery components and performance, the researchers also investigated the intercalation of the electrolyte compounds into the graphite with density functional theory calculations and experiments. The team found that by adding LiPF6, the electrolyte is stabilized against anodic oxidation. The battery showed a surprisingly high midpoint discharge voltage of 2.2 V and a wide electrochemical window of the electrolyte of 4 V. The high coulombic efficiency of 99.64 % is the highest reported for Zn-graphite batteries so far. No dendrite formation was observed and the battery retained 97.5 % of its capacity after long-term cycling. The experiments and calculations revealed that Zn and also both anions from the electrolyte are intercalated into the graphite. According to the researchers, this research provides new interesting impulses for research on battery materials.
- A High‐Voltage, Dendrite‐Free, and Durable Zn–Graphite Battery,
Gang Wang, Benjamin Kohn, Ulrich Scheler, Faxing Wang, Steffen Oswald, Markus Löffler, Deming Tan, Panpan Zhang, Jian Zhang, Xinliang Feng,
Advanced Materials 2019.
https://doi.org/10.1002/adma.201905681