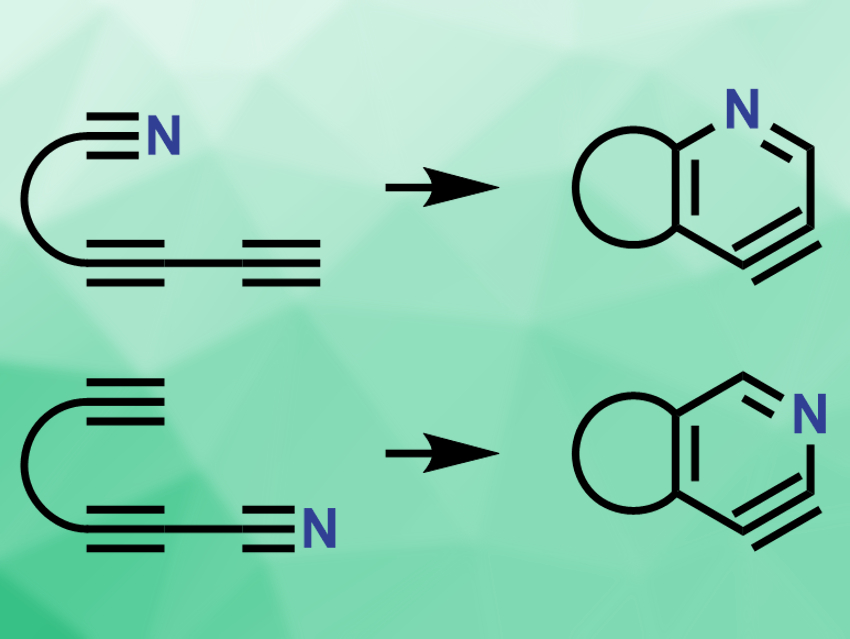
Hexadehydro-Diels–Alder (HDDA) reactions can be used to make benzynes from triynes. The triyne substrates for these reactions contain a conjugated 1,3-diyne and a tethered alkyne. Benzynes, or arynes, are reactive intermediates derived from arynes by removing two substituents.
Severin K. Thompson and Thomas R. Hoye, University of Minnesota, Minneapolis, USA, have developed the aza-variant of this reaction, i.e., aza-hexadehydro-Diels–Alder reactions (pictured). The team replaced a carbon atom in the substrate with a nitrogen atom, either at the tethered group or at the conjugated 1,3-diyne group. The resulting substrates are diyne nitriles. The substrates are heated in o-dichlorobenzene to provide the Diels–Alder products. In this case, the products are pyridynes, the nitrogen analogues of benzynes.
Depending on the location of the nitrile group in the substrate, the reaction gives either 3,4-pyridynes (pictured top) or 2,3-pyridynes (pictured bottom). These reactive intermediates can then be trapped by a variety of reagents, e.g., by a tert-butyldimethylsilyl ether, furan, or acetic acid. The resulting stable products are functionalized pyridines. According to the researchers, the aza-HDDA reaction is slower than the all-carbon HDDA reaction, but provides a new way to synthesize substituted pyridine compounds.
- The Aza-hexadehydro-Diels–Alder Reaction,
Severin K. Thompson, Thomas R. Hoye,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b11243