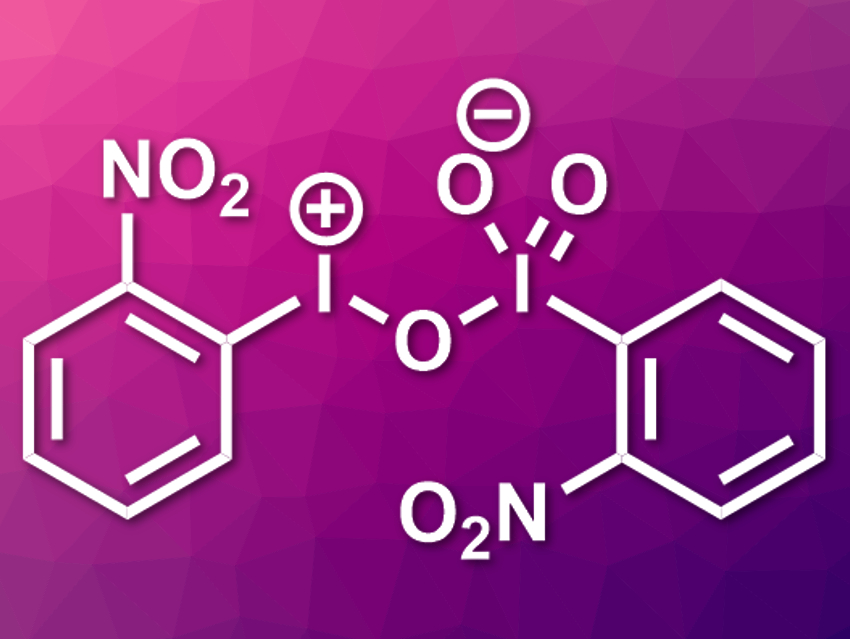
Hypervalent iodine reagents are useful in organic chemistry. Existing compounds of this type contain either iodine(III) or iodine(V).
Yunfei Du, Tianjin University, China, and colleagues have synthesized the first mixed hypervalent iodine compound which contains both I(III) and I(V) units (pictured). The team originally wanted to synthesize o-nitroiodoxybenzene (O2N–C6H4–IO2), but did not obtain the product. In the course of optimizing the reaction, the researchers accidentally found the new mixed reactant. It is produced as an orange solid when o-nitroiodobenzene reacts with meta-chloroperoxybenzoic acid (mCPBA) in acetic acid at 60 °C.
The compound was characterized using single-crystal X-ray crystallography and the bond situation was investigated using density functional theory (DFT) calculations. The team found that the nitro groups interact with the iodine atoms and stabilize the molecule. The new oxidant can be used for the synthesis of 2-unsubstituted 2H-azirines from enamines. This reaction cannot be achieved using traditional hypervalent iodine reagents.
- A New Hypervalent Iodine(III/V) Oxidant and Its Application to the Synthesis of 2H-Azirines,
Guangtao Zhang, Yuanxun Wang, Jun Xu, Jiyun Sun, Fengxia Sun, Yilin Zhang, Chenglin Zhang, Yunfei Du,
Chem. Sci. 2020.
https://doi.org/10.1039/c9sc05536c


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
