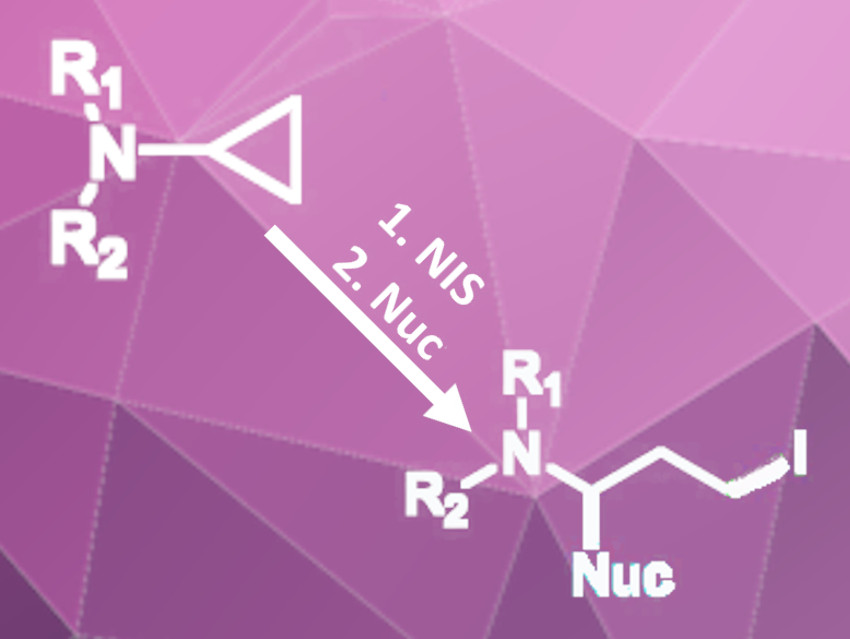
Cyclopropylamines can be used as synthetic building blocks for a variety of nitrogen-containing molecules. This is due to the high reactivity of the strained three-membered ring. The ring strain leads to a unique bonding situation and gives some amount of π-character to the bonds within the ring. Therefore, the cyclopropyl group can act as a π-donor and react with electrophiles, such as protons or electrophilic halogen sources.
Nan Zheng and Qile Wang, University of Arkansas, Fayetteville, USA, have developed a 1,3-difunctionalization of cyclopropyl amines (pictured) using N-iodosuccinimide (NIS) as an electrophilic iodine source and trimethylsilyl cyanide (TMS-CN), succinimide, or indole as nucleophiles. The reaction of various arylated cyclopropylamines with NIS and TMS-CN in diethyl ether containing one equivalent of water gives the disubstituted, ring-opened products in moderate to excellent yields.
The water was added to improve selectivity because a succinimide-substituted byproduct was formed. Because of the formation of this byproduct, the researchers tested the reaction in dichloromethane without water with succinimide or indole as the nucleophile instead of TMS-CN. This variant gave succinimide- or indole-substituted iodinated products in good to excellent yields.
The process was carried out under aerobic conditions and is compatible with substrates carrying functional groups that could react with radical intermediates. Therefore, the researchers assume that the cyclopropyl amine reacts with the electrophilic iodine species in an SN2-like two-electron process. The resulting products could be further functionalized by replacing the iodide group with other nucleophiles.
- Difunctionalization of Cyclopropyl Amines with N-Iodosuccinimide (NIS) or in Situ Formed Cyanogen Iodide (ICN),
Qile Wang, Nan Zheng,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b03922