Etoposide (pictured) is used as a drug against different types of cancer. It is on the World Health Organization’s (WHO) list of essential medicines. The drug is currently produced semi-synthetically based on an extract from the Himalayan mayapple, which is considered endangered. There have been several etoposide shortages in the past. Thus, an alternative path to the drug is needed.
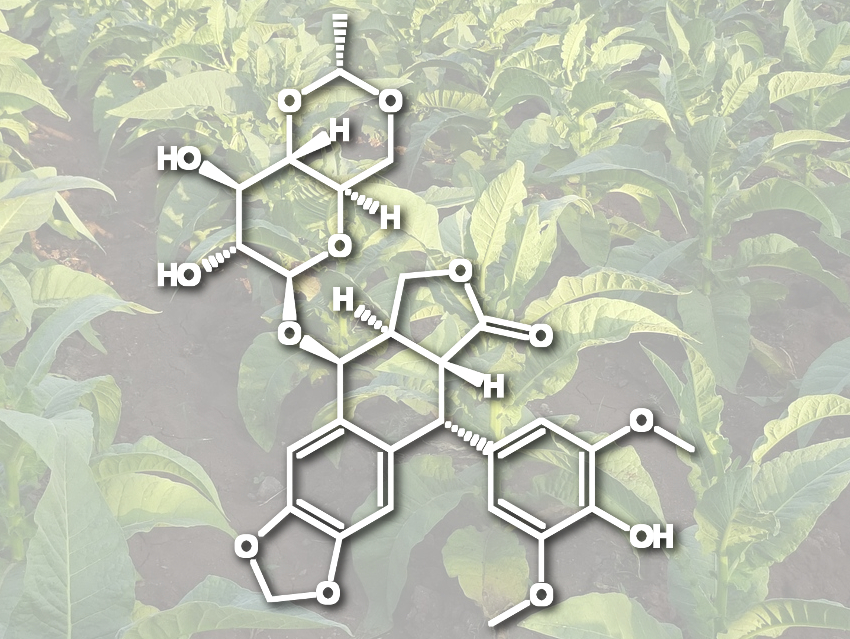
Elizabeth S. Sattely, Stanford University, CA, USA, and colleagues have developed a way to produce (−)-deoxypodophyllotoxin, a precursor to etoposide, on a milligram scale. For this, the team used an engineered biosynthetic pathway in tobacco plants. For this “foreign-host” approach, the researchers used an Agrobacterium strain to transport the 16 necessary genes for the synthesis into tobacco leaves. These genes cause a higher production of coniferyl alcohol and encode the enzymes needed to convert it to the product. The coniferyl alcohol is dimerized to give (+)-pinoresinol, which is further processed to the desired (−)-deoxypodophyllotoxin.
The team achieved yields of 4.3 mg (−)-deoxypodophyllotoxin per gram of dry plant weight. According to the researchers, the synthesis is the longest natural product pathway reconstituted in a plant heterologous host so far. The work could help to synthesize analogues of etoposide, ensure its supply, and promote the development of tobacco as a platform for the production of other plant-derived drugs.
- Total Biosynthesis for Milligram-Scale Production of Etoposide Intermediates in a Plant Chassis,
Bailey J. Schultz, Seung Yeon Kim, Warren Lau, Elizabeth S. Sattely,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b10717
Also of Interest
- The Chemistry of Tobacco,
Sabine Streller and Klaus Roth,
ChemViews Mag. 2014.
https://doi.org/10.1002/chemv.201400096