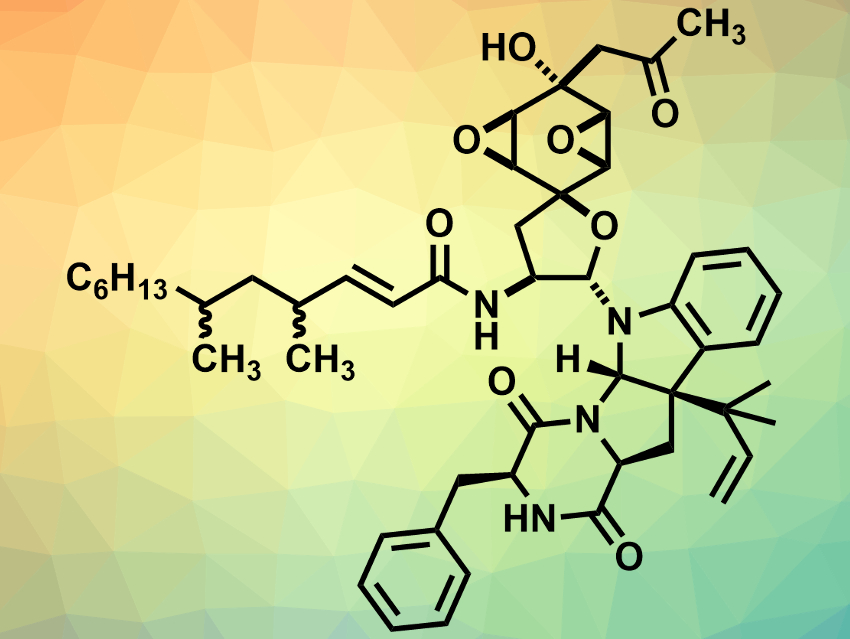
Penicimutanin A (pictured) was isolated from a marine fungus. Its absolute configuration had not been determined so far. In addition, some of its derivatives show useful biological activity, but the amounts that are available are too small for comprehensive biological studies.
Tao Xu, Ocean University of China and Pilot National Laboratory for Marine Science and Technology, Qingdao, and colleagues have performed the first total synthesis of penicimutanin A and determined its absolute configuration. The synthesis is concise, with ten steps (longest linear sequence). The team started from commercially available (S)-tryptophan and (S)-tyrosine derivatives. Key steps of the synthesis include an electro-oxidative dearomatization, a bisoxirane-directed intermolecular aldol reaction, and diastereoselective one-step Meerwein-Eschenmoser-Claisen rearrangement.
The team also synthesized the related compounds penicimutanolone, fructigenine A, and penicimutatin. The syntheses should allow the biological profiling of this family of compounds.
- Total Synthesis of (‒)-Penicimutanin A and the Related Congeners,
Haiyong Yu, Yan Zong, Tao Xu,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc05252f