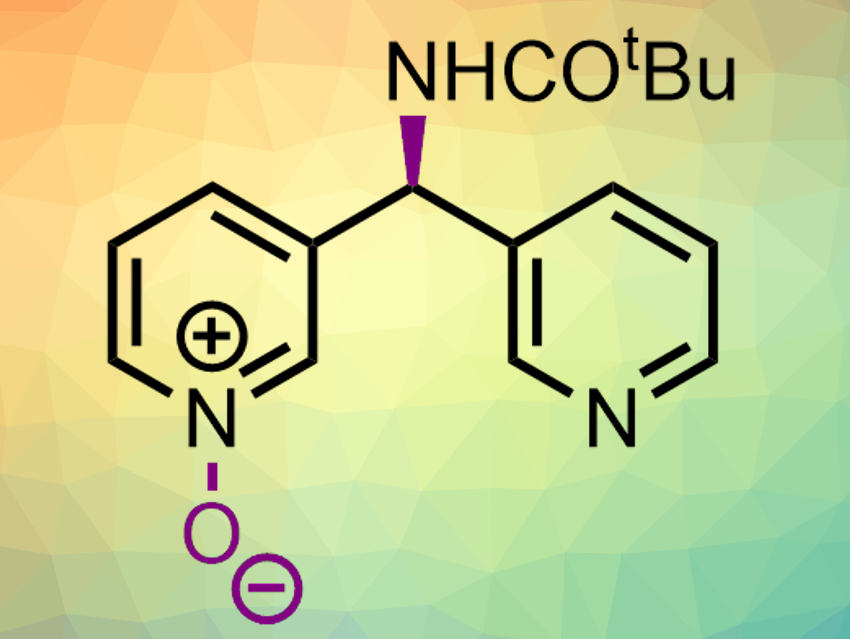
Chiral pyridine derivatives are part of many bioactive molecules. Thus, the enantioselective synthesis of substituted pyridines is important. Often, this requires chiral auxiliaries or building blocks, but asymmetric catalysis can also be used.
Scott J. Miller, Yale University, New Haven, CT, USA, and colleagues have developed a catalytic enantioselective pyridine N-oxidation that can be used for the desymmetrization of bispyridine substrates (product example pictured). The team used aspartic-acid-containing peptides as biomolecule-inspired catalysts. The aspartic acid group in these chiral catalysts can cycle between the acid and a peroxy acid form in the presence of H2O2. The substrates have a prochiral substituent that can form hydrogen bonds with the catalyst. The peroxy acid then converts the substrates to pyridine N-oxides in an enantioselective manner.
This approach allowed the team to convert a range of substituted bispyridines into chiral pyridine N-oxides in moderate to good yields and with enantiomeric ratios up to 99:1. The resulting products can be further functionalized. The reaction can also be used for the enantioselective oxidation of existing pyridine-containing drugs to provide new derivatives.
- Catalytic Enantioselective Pyridine N-Oxidation,
Sheng-Ying Hsieh, Yu Tang, Simone Crotti, Elizabeth A. Stone, Scott J. Miller,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b10414