Biomass, such as lignocellulose from wood, could be a sustainable source of chemical feedstocks. However, lignocellulose is rarely used for the production of polymers because it is difficult to convert to useful monomers.
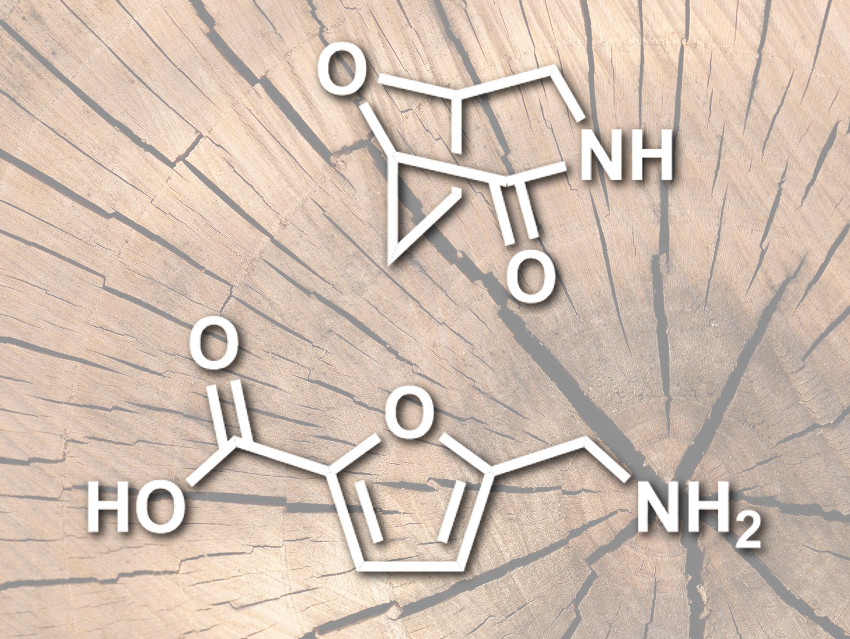
Andrew W. Lankenau and Matthew W. Kanan, Stanford University, CA, USA, have converted furfurylamine, which is industrially produced from lignocellulose, into monomers for polyamides (pictured). The monomers, the lactam 8-oxa-3-azabicyclo[3.2.1]octan-2-one (pictured top) and the amino acid 5-(aminomethyl)-furan-2-carboxylic acid (pictured bottom) are produced by a carbonate-promoted C–H carboxylation of furfurylamine. The furfurylamine was combined with Cs2CO3 under 60 bar of CO2 at 170 °C to give a carboxylated intermediate. This intermediate was converted to the desired furan-based amino acid using HCl. The lactam monomer can be obtained from this amino acid by hydrogenation.
The reaction requires no protecting groups, uses low-cost solvents (water and acetic acid), and the Cs ions can be recovered and reused. According to the researchers, this could allow a scalable monomer production.
- Polyamide Monomers Via Carbonate-Promoted C–H Carboxylation of Furfurylamine,
Andrew W Lankenau, Matthew Kanan,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc04460d
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

