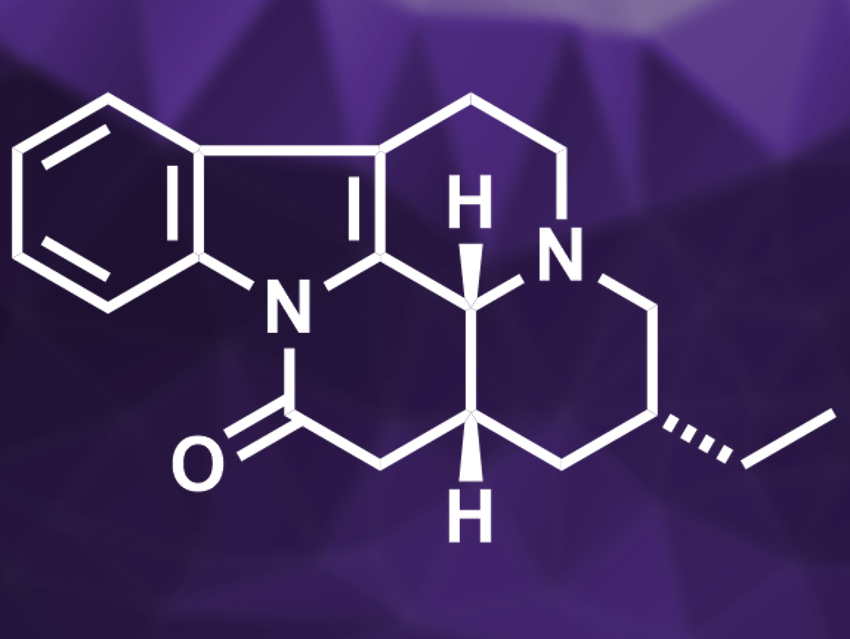
Tacamonine (pictured) is a pentacyclic indole alkaloid found in a type of dogbane plant. Alkaloids that are isomeric to tacamonine have useful biological activities, but tacamonine itself and its closely related compounds have not been well studied, so far. Most existing syntheses of tacamonine have problems with stereoselectivity and/or a large number of steps.
Roger Hunter, University of Cape Town, South Africa, and colleagues have developed a concise synthesis of (+)-tacamonine. The team used a commercially available oxazolidinone as a chiral auxiliary to prepare a chiral acid from butyryl chloride and allyl bromide. The resulting intermediate was coupled with dihydro-β-carboline. In the following key step, a stereoselective radical cyclization of the obtained 1-phenylsulfanyl tetrahydro-β-carboline with an ester-functionalized side chain is performed. In this step, two stereocenters are formed selectively based on a single stereocenter in the side chain. A final cyclization forms the amide bond and gives the desired product.
In total, the synthesis has 10 steps (longest linear sequence) and gives 24.5 % overall yield. According to the researchers, it is the shortest and most efficient asymmetric synthesis of (+)-tacamonine so far. The amount of product obtained should allow biological studies of tacamonine and its derivatives.
- Synthesis of (+)-Tacamonine via Stereoselective Radical Cyclization,
Myles W. Smith, Jasmin Ferreira, Roger Hunter, Gerhard A. Venter, Hong Su,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b03308