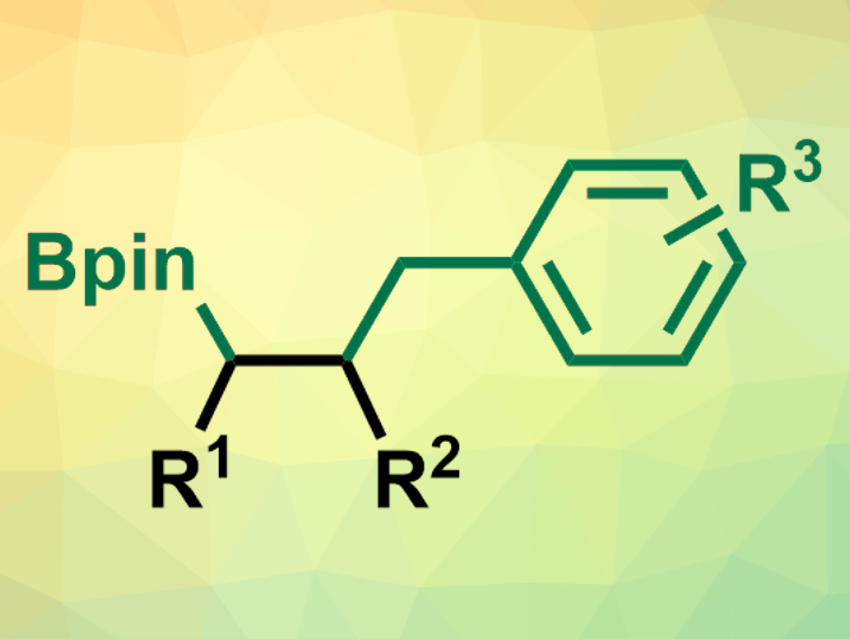
The 1,2-carboboration of alkenes is interesting for synthetic organic chemistry because it forms a C–C bond and a reactive C–B bond in one step. 1,2-Benzylborations, in particular, could lead to many useful synthetic intermediates. This type of reaction, however, is challenging for unactivated alkenes.
M. Kevin Brown and colleagues, Indiana University, Bloomington, USA, have developed a nickel-catalyzed 1,2-benzylboration of 1,2-disubstituted unactivated alkenes. The team used Ni(COD)2 (COD = 1,5-cyclooctadiene) as the catalyst, sodium tert-pentoxide as a base, and (Bpin)2 (bis(pinacolato)diboron) as a diboron reagent together with a variety of substituted benzylchlorides to functionalize different 1,2-disubstituted alkenes in dimethylacetamide (DMA) at 30 °C. Cyclopentenes and related heterocycles are also suitable substrates.
The desired products (pictured) were obtained in moderate yields and excellent diastereoselectivity. The team used the method to prepared biologically active compounds, which demonstrates its potential. The approach extends the substrate scope of nickel-catalyzed carboboration reactions.
- Ni-catalyzed 1,2-benzylboration of 1,2-disubstituted unactivated alkenes,
Seewon Joung, Allison M. Bergmann, M. Kevin Brown,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc04199k