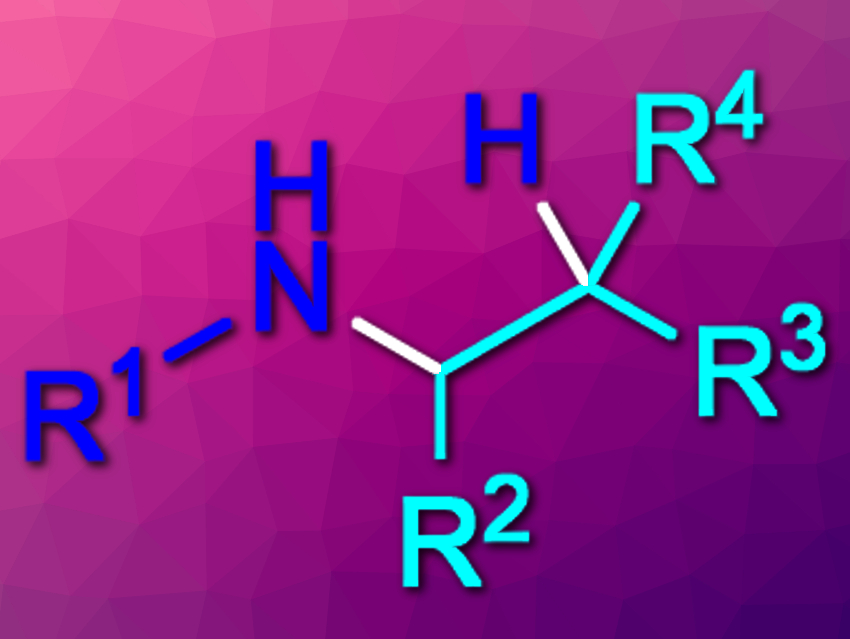
Amines are commonly used in, e.g., pharmaceutical chemistry. They can, for example, be synthesized via the hydroamination of alkenes. Intermolecular hydroaminations of unactivated alkenes, however, can be challenging. In addition, existing methods for this type of reaction usually give the Markovnikov products (which are aminated at the higher-substituted carbon). Until now, there had been no general method for the intermolecular anti-Markovnikov hydroamination of unactivated alkenes with primary alkyl amines.
Robert R. Knowles, Princeton University, NJ, USA, and colleagues have developed a photocatalytic method that gives the anti-Markovnikov hydroamination products(pictured) from unactivated alkenes and primary alkyl amines. The team used [Ir(dF(CF3)ppy)2(4,4′-d(CF3)bpy)]PF6 as a photocatalyst (dF(CF3)ppy = 2-(2,4-difluorophenyl)-5-
(trifluoromethyl)pyridine; 4,4′-d(CF3)bpy = 4,4′-bis(trifluoromethyl)-2,2′-bipyridine) and 2,4,6-triisopropylbenzenethiol (TRIP thiol) as a H-atom transfer (HAT) catalyst. The reaction was performed in dioxane under a blue light-emitting diode (LED) at room temperature.
The desired products were obtained in moderate to excellent yields. The reaction provides high selectivities for the secondary over the tertiary amine products, i.e., it avoids over-alkylation. The team observed no Markovnikov addition products for all but one of the tested substrates. According to the researchers, the transformation proceeds via aminium radical cation (ARC) intermediates.
- Anti-Markovnikov Hydroamination of Unactivated Alkenes with Primary Alkyl Amines,
David C. Miller, Jacob M. Ganley, Andrew J. Musacchio, Trevor C. Sherwood, William R. Ewing, Robert R. Knowles,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b08746