Malaria and other insect-borne diseases are a dangerous health issue in many parts of the world. Today, pyrethroid insecticides are the major agents used against disease-carrying insects. Resistance is on the rise, however. Therefore, the use of DDT (dichlorodiphenyltrichloroethane), previously banned due to its persistence and environmental and health hazards, is being reconsidered for indoor applications. However, some insect populations have already developed resistance against this agent, as well.
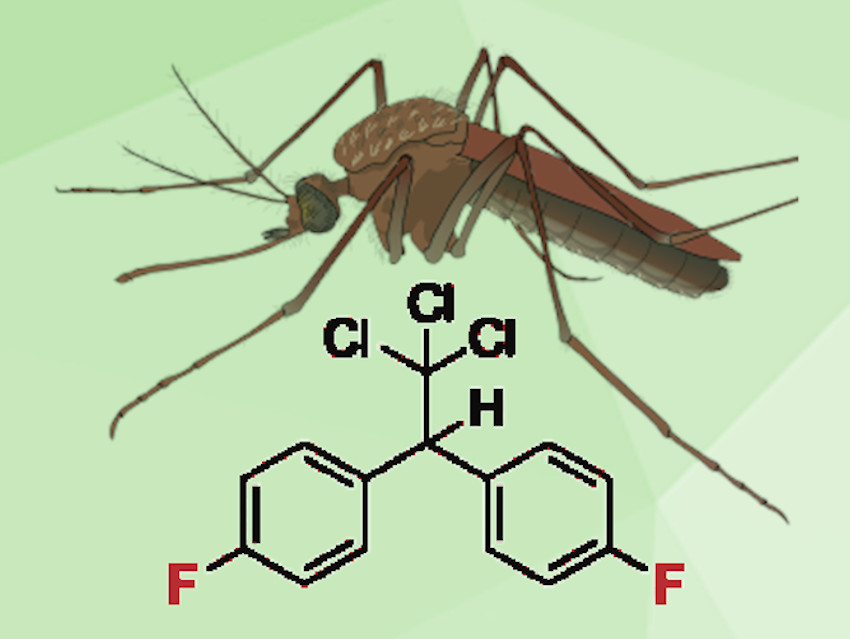
During World War II, a DDT analogue carrying a fluorine atom on each phenyl group instead of DDT’s chlorine was developed in Germany. This compound, DFDT (pictured), showed faster insecticidal action and lower mammalian toxicity but was abandoned after the end of the war due to the efficiency and easy availability of DDT.
Michael D. Ward, Bart Kahr, New York University, USA, and colleagues have synthesized DFDT as well as a chiral derivative carrying one fluoro- and one chloro group and analyzed the compounds’ behavior in the solid state. The team grew single crystals of these compounds, analyzed them by X-ray diffraction, and tested the insecticidal effects of crystalline and amorphous forms of the two fluorinated agents and DDT against Drosophila flies, Anopheles mosquitoes (which can transmit malaria) and Aedes mosquitoes (which can transmit, e.g., the Zika virus).
The results showed that the amorphous forms of the three insecticides are more efficient at killing the insects than their crystalline phases. Amorphous DFDT has the fastest insecticidal action. The researchers suggest the use of DFDT as an alternative to pyrethroids and DDT. DFDT might be less prone to resistance development due to its fast action. The environmental effects of DFDT still need to be assessed, but its lower mammalian toxicity compared to DDT could provide an advantage for indoor applications.
- Manipulating Solid Forms of Contact Insecticides for Infectious Disease Prevention,
Xiaolong Zhu, Chunhua T. Hu, Jingxiang Yang, Leo A. Joyce, Mengdi Qiu, Michael D. Ward, Bart Kahr,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b08125