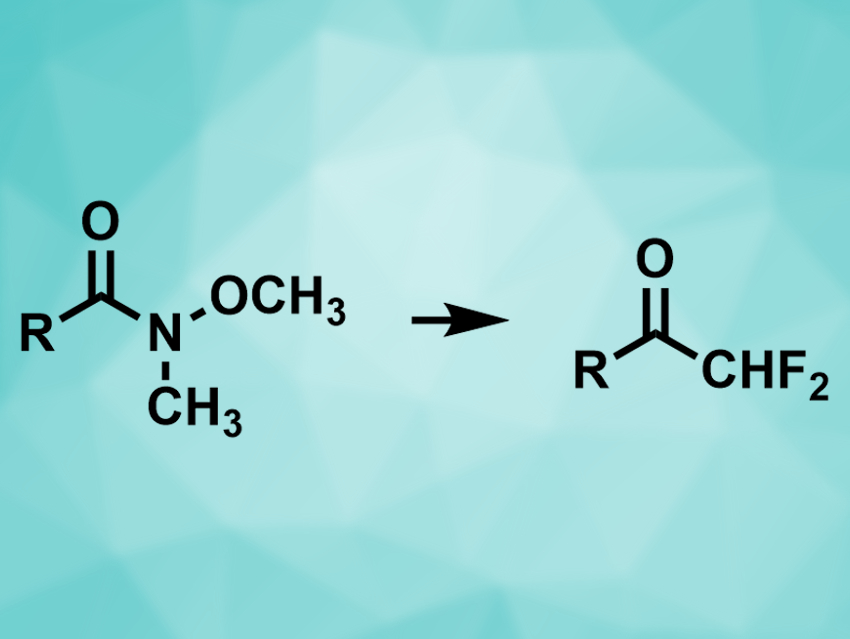
Introducing difluoromethyl groups can be useful to improve the properties of organic molecules, particularly for use in pharmaceutical chemistry. It can also change a compound’s reactivity, e.g., make ketones more electrophilic. The groups can either be built by successive fluorinations—which can cause selectivity problems and lead to a mixture of products—or by using a preexisting difluoromethyl building block.
Vittorio Pace, University of Vienna, Austria, and colleagues have converted Weinreb amides into difluoromethylketones, using the commercially available TMSCHF2 (TMS = trimethylsilyl) as a difluoromethyl transfer agent. The team reacted a range of different Weinreb amides (pictured left) with TMSCHF2 in the presence of potassium tert-pentyloxide at 0 °C. The desired products (pictured right) were obtained in excellent yields.
TMSCHF2 acts as a nucleophilic difluoromethyl transfer agent in the reaction after activation by the alkoxide. The method tolerates a variety of functional groups, e.g., ketones, esters, other amides, and nitro- and nitrile groups. The resulting ketones are useful substrates for further transformations.
- Direct and Chemoselective Synthesis of Tertiary Difluoroketones via Weinreb Amide Homologation with a CHF2-Carbene Equivalent,
Margherita Miele, Andrea Citarella, Nicola Micale, Wolfgang Holzer, Vittorio Pace,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b03024