Metal carbyne complexes, i.e., species with a metal–carbon triple bond (M≡CR), are common in alkyne metathesis. However, their general use for carbyne transfer has not been well explored. This is due to a lack of carbyne souces. This problem could be solved by using metal-carbynoids as equivalents instead of metal-carbynes. A metal–carbene complex with a good leaving group, for example, could act as such an equivalent.
Marcos G. Suero, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, have found that rhodium-carbynoids can be synthesized from a carbyne precursor with a hypervalent iodine moiety, a diazo group, and a CO2Et group. These carbyne sources were subjected to a selective diazo activation in the presence of paddlewheel dirhodium complexes of the type L4Rh2 (L = acetate, trifluoroacetate, heptafluorobutyrate, etc.). Due to the outstanding leaving group ability of the hypervalent iodine unit, the resulting rhodium-carbynoids act like a monovalent cationic carbyne.
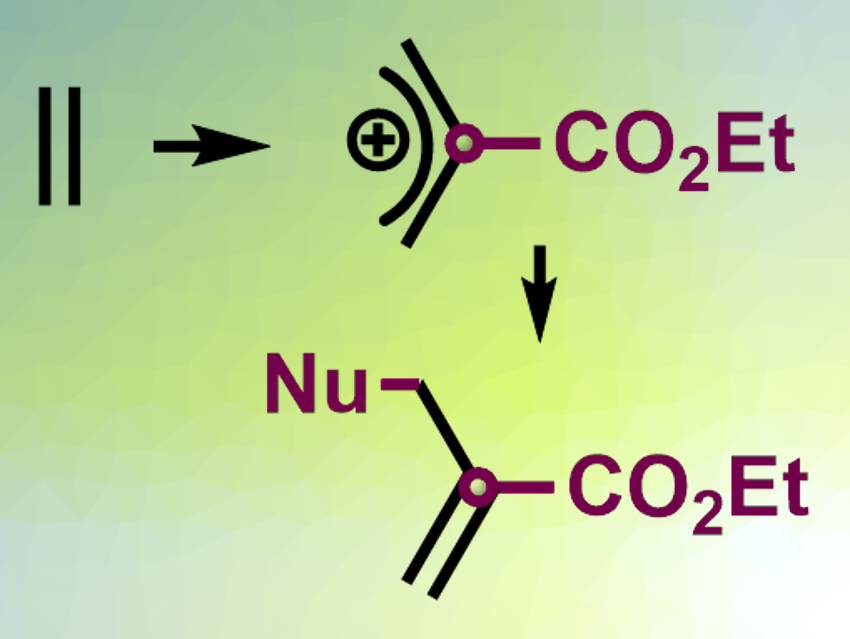
The Rh-carbynoids can be used as a catalyst to insert a single carbon into the middle of the C(sp2)–C(sp2) bond of an alkene (pictured top). The resulting allylic cation can react with a nucleophile to give substituted allyl species (pictured bottom). According to the researchers, the insertion reaction proceeds via a hypervalent-iodine-substituted cyclopropyl intermediate. The reaction can be used to extend the carbon skeleton of organic compounds and build complex structures.
- Catalytic Cleavage of C(sp2)–C(sp2) Bonds with Rh-Carbynoids,
Zhaofeng Wang, Liyin Jiang, Pau Sarró, Marcos G. Suero,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b08632