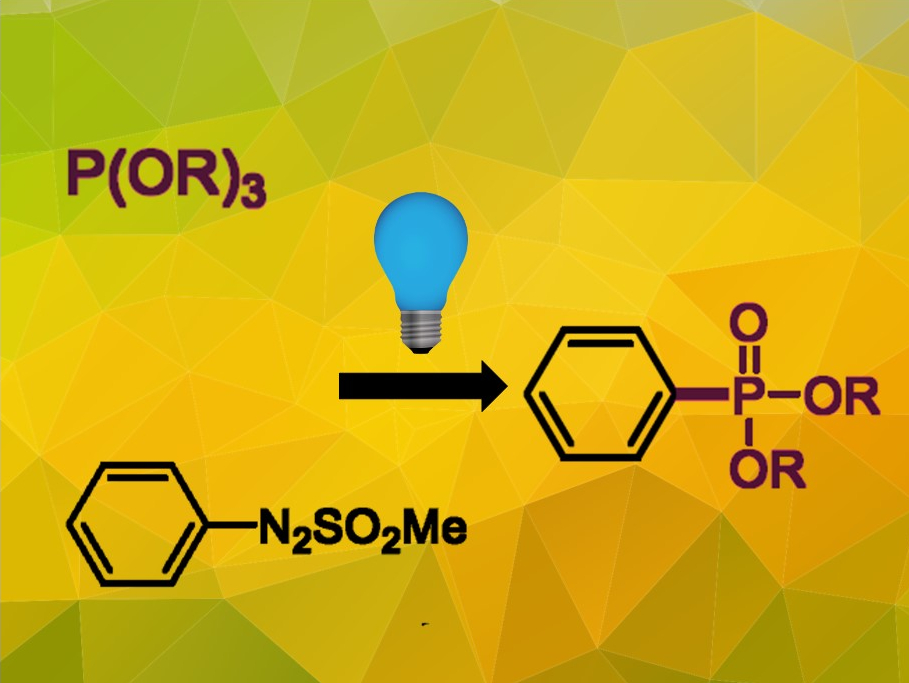
Organic phosphonates are an important class of compounds with a wide range of applications in materials chemistry, medicinal chemistry, crop protection, and organic synthesis. Most established synthesis procedures require transition metals, organometallic reagents, or other additives.
Di Qiu, Tianjin Normal University, China, Stefano Protti, University of Pavia, Italy, and colleagues have developed a visible-light-driven synthesis route for aryl phosphonates, using arylazo sulfones as the starting material. The arylazo sulfones (pictured bottom left) were prepared from the arenediazonium salts and sodium methanesulfinate in dichloromethane at room temperature. The isolated arylazo sulfones were reacted with a phosphite (pictured top left) under the irradiation of a blue light-emitting diode (LED) over 24 hours in acetonitrile under a nitrogen atmosphere. The desired phosphonates (pictured right) were obtained in moderate to good yields.
The addition of the stable organic radical 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) prevents the reaction, which indicates a radical-driven mechanism. The method provides a photocatalyst- and additive-free synthesis path for aryl phosphonates. The reaction tolerates a wide range of functional groups at the aryl moiety, including electron-donating groups which cause difficulties in other phosphonate syntheses.
- Visible Light‐Driven, Photocatalyst‐Free Arbuzov‐Like Reaction via Arylazo Sulfones,
Di Qiu, Chang Lian, Jinshan Mao, Yi Ding, Zerong Liu, Liyan Wei, Maurizio Fagnoni, Stefano Protti,
Adv. Synth. Catal. 2019.
https://doi.org/10.1002/adsc.201900953