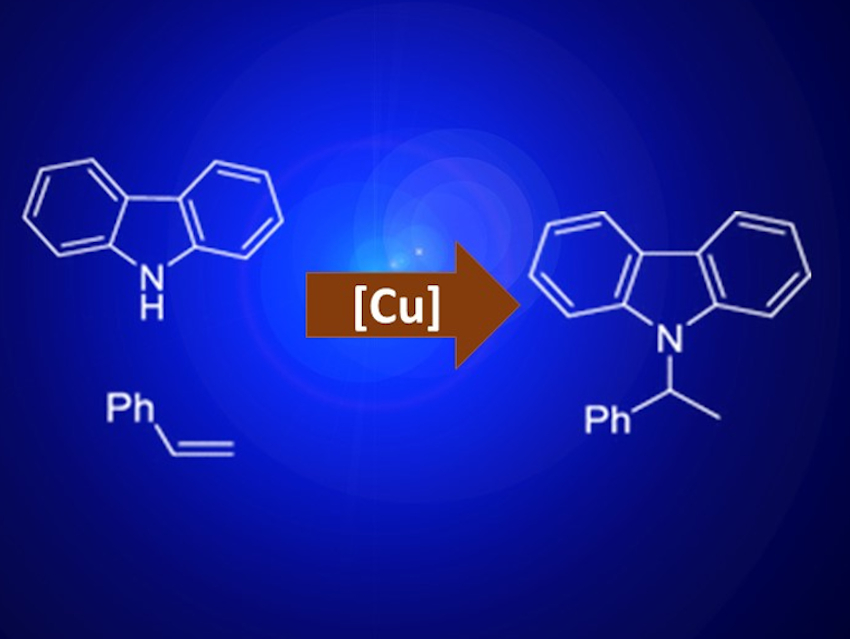
Amines are an important class of organic molecules. They are part of many biomolecules and biologically active compounds and often serve as synthetic intermediates. Hydroamination is an elegant route to new amines because it is atom-economical and can make use of commercially available starting materials.
Guozhu Zhang, Chinese Academy of Sciences, Shanghai, and colleagues have developed a hydroamination protocol for styrene derivatives using copper catalysis and irradiation from a blue light-emitting diode (LED) to give the Markovnikov product. The researchers tested various copper salts, with Cu(CH3CN)4PF6 and Cu(OTf)2 giving the highest yields. Acetonitrile was used as the solvent and LiOtBu served as a base. All reactions were carried out at room temperature under nitrogen.
When indoline or aniline derivatives were used as amine starting material instead of, e.g., 9H-carbazole, the conversion following the standard procedure was not successful. This is probably due to the reduced photoactivity of the smaller π-system. In these cases, the addition of 1,1-bi-2-naphtol (BINOL) as a co-catalyst led to good yields. BINOL activates the catalyst by forming a photoactive Cu complex. A wide variety of para-substituted aniline derivatives could be converted to the corresponding hydroamination products using this method.
The approach allows the conversion of a wide range of styrene derivatives and aromatic amines carrying a variety of functional groups. The products were obtained in good yields under comparably mild conditions.
- Visible-Light-Induced Copper-Catalyzed Intermolecular Markovnikov Hydroamination of Alkenes,
Yang Xiong, Guozhu Zhang,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b02863