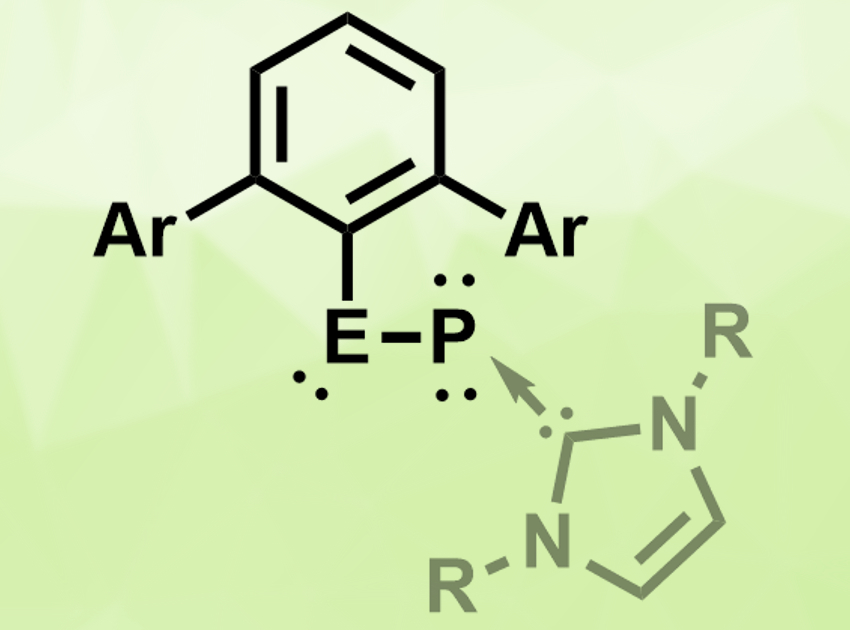
Nitriles are organic compounds with a −C≡N functional group. Heavier analogues of such groups—for example, −E≡P (E = Si, Ge, Sn, Pb)—could have interesting properties and applications in, e.g., synthesis or catalysis.
Shigeyoshi Inoue, Technical University Munich, Garching, Germany, and colleagues have synthesized heavier analogues of nitriles in the form of monomeric tetrylene–phosphinidenes, stabilized by N-heterocyclic carbenes (NHCs). The compounds of the type MesTerEP(IDipp) (pictured, E = Ge, Sn; MesTer = 2,6-Mes2C6H3, IDipp = C([N-(2,6-iPr2C6H4)CH]2) were obtained by reacting the germylene or stannylene chloride dimer [MesTerECl]2 with the trimethylsilyl phosphinidene complex (IDipp)PSiMe3 under elimination of trimethylsilyl chloride. The yields were 82 % for the germanium compound and 72 % for the tin compound.
The products were characterized using 31P and 119Sn NMR spectroscopy and single-crystal X-ray diffraction. Quantum-chemical calculations were performed to better understand the bonding situation. The compounds have bent structures with bond angles around Ge or Sn close to 90°. The E–P bond lengths, as well as calculated bond indices, suggest a multiple-bond character. The E–P bonds are reactive towards, e.g., ketenes, and the tin compound shows catalytic activity in the hydroboration of aromatic aldehydes and ketones.
- N-Heterocyclic Carbene-Stabilized Germanium and Tin Analogues of Heavier Nitriles: Synthesis, Reactivity, and Catalytic Application,
Vitaly Nesterov, Ramona Baierl, Franziska Hanusch, Arturo Espinosa Ferao, Shigeyoshi Inoue,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b08741