Vinylsilanes are useful intermediates in organic synthesis. They can be prepared, e.g., by the hydrosilylation of alkynes. There are several methods for the synthesis of β-vinylsilanes, while paths to α-vinylsilanes are rarer. Some of the existing methods for the synthesis of α-vinylsilanes suffer from unstable or expensive catalysts, which hampers their industrial application.
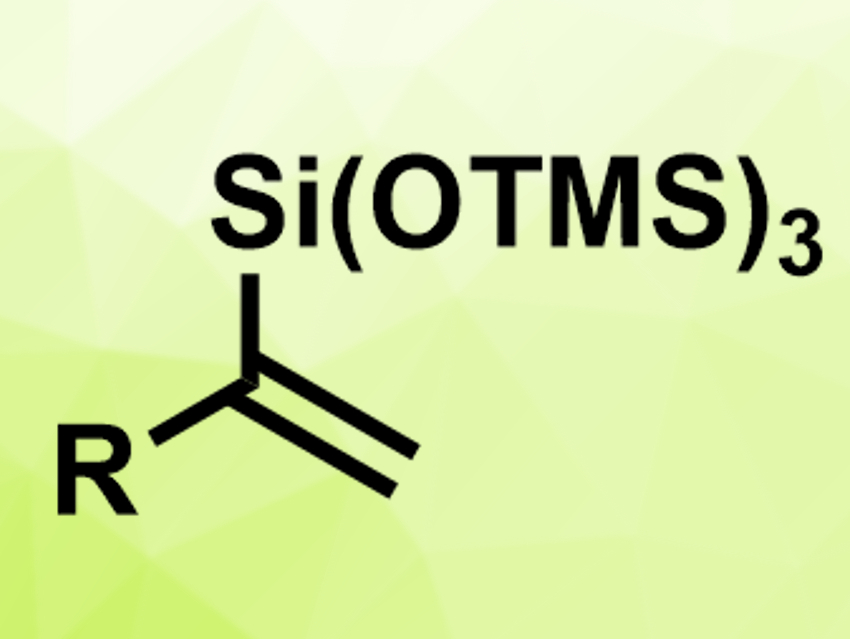
Shengtao Ding, Beijing University of Chemical Technology, China, and colleagues have developed an iridium-catalyzed hydrosilylation of terminal alkynes with α-regioselectivity. The team used the simple catalyst [Ir(μ-Cl)(cod)]2 (cod = 1,5-cyclooctadiene) and the bulky trimethylsilyl-protected trihydroxysilane (TMSO)3SiH to convert a variety of terminal alkynes to α-vinylsilanes (pictured) in acetonitrile at room temperature.
The reaction proceeds with good regio- and stereoselectivity and provides high yields. According to the team, it is the first iridium-catalyzed hydrosilylation of terminal alkynes with excellent α-selectivity. The (TMSO)3Si group can be converted into other useful silyl groups.
- Iridium-catalyzed Markovnikov hydrosilylation of terminal alkynes achieved by using a trimethylsilyl-protected trihydroxysilane,
Xingze Xie, Xueyan Zhang, Weiwei Gao, Congcong Meng, Xiaojun Wang, Shengtao Ding,
Commun. Chem. 2019.
https://doi.org/10.1038/s42004-019-0206-4