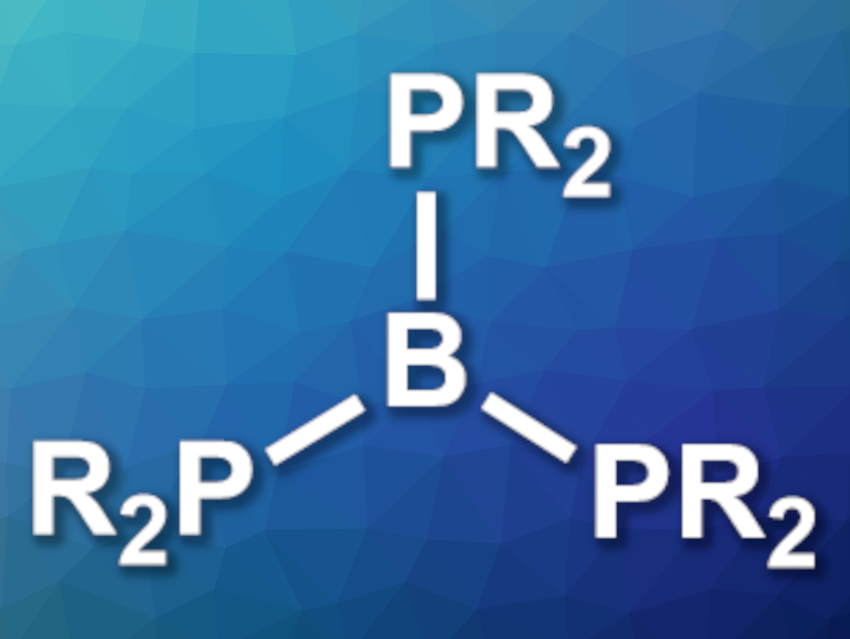
Compounds that contain directly linked phosphorus and boron atoms can be useful, e.g., in the activation of small molecules. Phosphinoboranes (R2P–BR’2) and diphosphinoboranes (BR(PR’2)2) are examples of this type of compound. There are known phosphinoboranes and diphosphinoboranes, but no fully characterized monomeric species of the type B(PR2)3 (pictured) had been reported so far. Only a single example had been known (B(PMes2)2PMe2), and its structure was confirmed only by NMR spectroscopy [1].
Rafał Grubba, Gdańsk University of Technology, Poland, and colleagues have prepared a series of the first fully characterized triphosphinoboranes. To synthesize triphosphinoboranes, the team reacted BBr3 with an excess of lithium phosphides such as tBu2PLi, Cy2PLi, iPr2PLi, tBuPhPLi, or Ph2PLi. The triphosphinoborane B(PtBu2)3 could be obtained directly when three equivalents of the corresponding lithium phosphide were used. Other triphosphinoboranes with mixed substituents were obtained via a two-step process, in which the bromodiphosphinoborane BBr(PtBu2)2 was formed first and then reacted with a second lithium phosphide with less bulky substituents.
The structures of the triphosphinoboranes were confirmed by single-crystal X-ray diffraction. Based on the structures and the results of computational methods, the team found that the products can be divided into two types: one featuring delocalized π-bonding and one in which there is a localized P=B double bond and two P–B single bonds. According to the researchers, the products could be useful in the activation of small molecules.
- Monomeric Triphosphinoboranes: Intramolecular Lewis Acid–Base Interactions between Boron and Phosphorus Atoms,
Anna Ordyszewska, Natalia Szynkiewicz, Jarosław Chojnacki, Rafał Grubba,
Inorg. Chem. 2022.
https://doi.org/10.1021/acs.inorgchem.1c03618
Reference
- [1] The structure of a monomeric phosphinoborane: synthesis and x-ray crystal structure of (diphenylphosphino)dimesitylborane,
Xudong Feng, Marilyn M. Olmstead, Philip P. Power,
Inorg. Chem. 1986, 25, 26, 4615–4616.
https://doi.org/10.1021/ic00246a002