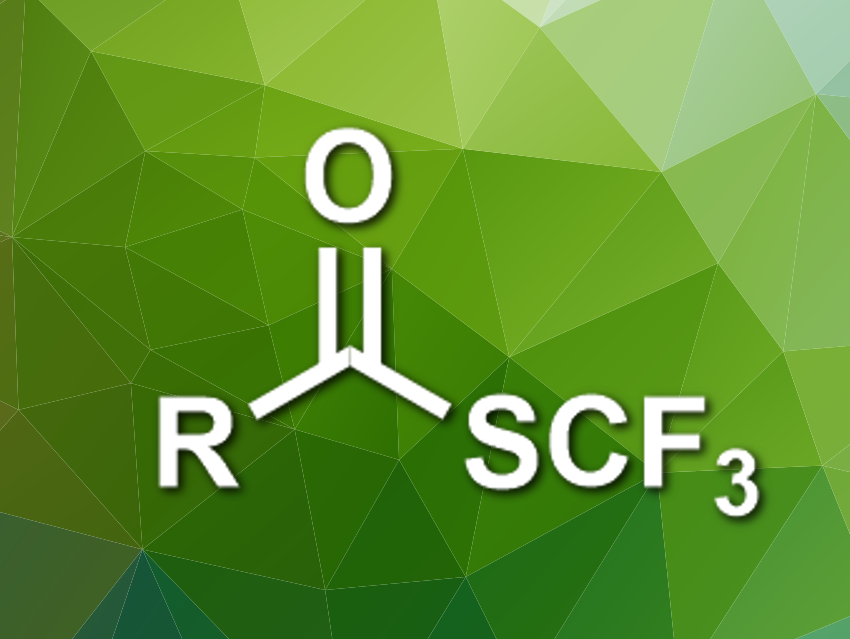
Organic compounds with fluorine substituents are useful, e.g., in pharmaceutical chemistry. The trifluoromethylthio group (SCF3), for example, has electron-withdrawing and lipophilic properties that are interesting for medicinal applications. This group occurs, e.g., in trifluoromethyl thioesters. However, known methods for the synthesis of these thioesters require toxic reagents or give stoichiometric amounts of metal-containing byproducts.
Xile Hu and colleagues, École Polytechnique Fédérale de Lausanne (EPFL), Switzerland, have developed a deoxygenative trifluoromethylthiolation that converts carboxylic acids into trifluoromethyl thioesters. The team used triphenylphosphine (PPh3) to activate the electrophilic trifluoromethylthiolating reagent N-(trifluoromethylthio)phthalimide. The resulting [F3CS–PPh3]+ ion reacts with the carboxylic acid to give an acyloxyphosphonium intermediate. Ph3P=O is then eliminated to give the desired trifluoromethyl thioesters.
The reaction was performed in the presence of FeCl3 as a Lewis acid in tetrahydrofuran (THF) at room temperature. The trifluoromethyl thioesters were obtained in moderate to excellent yields. The method has a good functional group tolerance, which makes it useful for the late-stage functionalization of drug-like molecules.
- Deoxygenative Trifluoromethylthiolation of Carboxylic Acids,
Runze Mao, Srikrishna Bera, Alexis Cheseaux, Xile Hu,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc3396c