Transition metals in complexes can shift between different redox states, which makes them useful in homogeneous catalysis. Noble metals have been used particularly often, but more cost-effective and sustainable transition metals such as iron, nickel, cobalt, or copper can also be useful in catalysis. Using inexpensive, non-toxic main group elements would be even better. However, it is challenging to replace a transition metal with a main group element in such reactions.
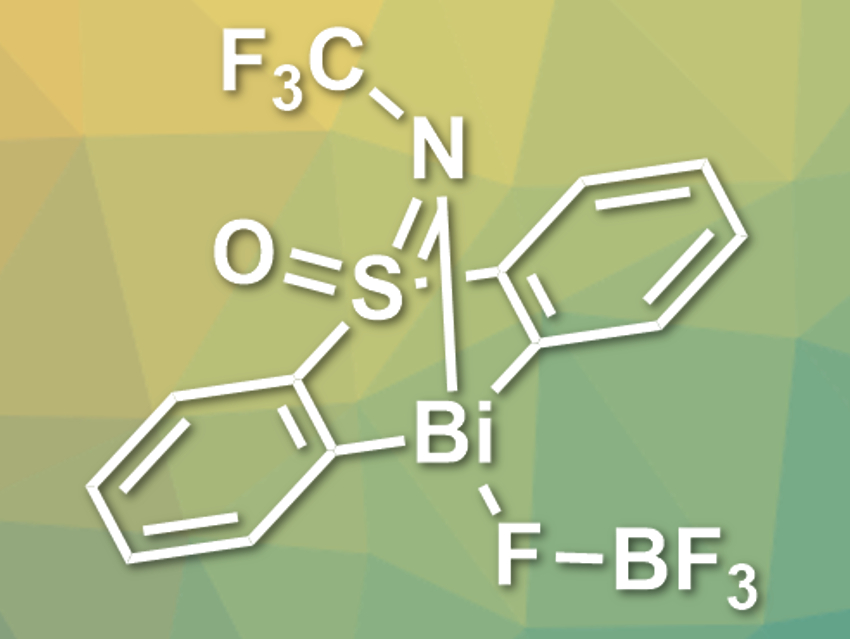
Josep Cornella and colleagues, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, have developed an unprecedented catalytic protocol for the oxidative fluorination of boronic acid derivatives using the redox couple Bi(III)/Bi(V). In this protocol, bismuth performs the same steps that are common in transition-metal catalysis, i.e., transmetallation, oxidative addition, and reductive elimination. The team used a rationally designed Bi compound as the catalyst (structure pictured).
The Bi-based catalyst can be used for the fluorination of arylboronic acid derivatives in good to excellent yields. The team used the catalyst together with NaF and an electrophilic fluorine source in CDCl3 at 90 °C. Yields were determined by 19F NMR. The researchers propose that the mechanism involves the transmetallation of the arylboronic acid derivative to the electrophilic Bi(III) center, followed by oxidation using the electrophilic fluorine source to give a high-valent Bi(V) compound, which decomposes while creating the desired C–F bond. According to the team, the results show that main-group elements could even outperform transition metals in certain fields of catalysis.
- Catalytic Fluorination of Boron Compounds at a Bismuth Redox Platform,
Josep Cornella, Markus Leutzsch, Feng Wang, Oriol Planas,
ChemRxiv 2019.
https://doi.org/10.26434/chemrxiv.9729143.v1The research has been published as a preprint and has not yet been peer-reviewed.