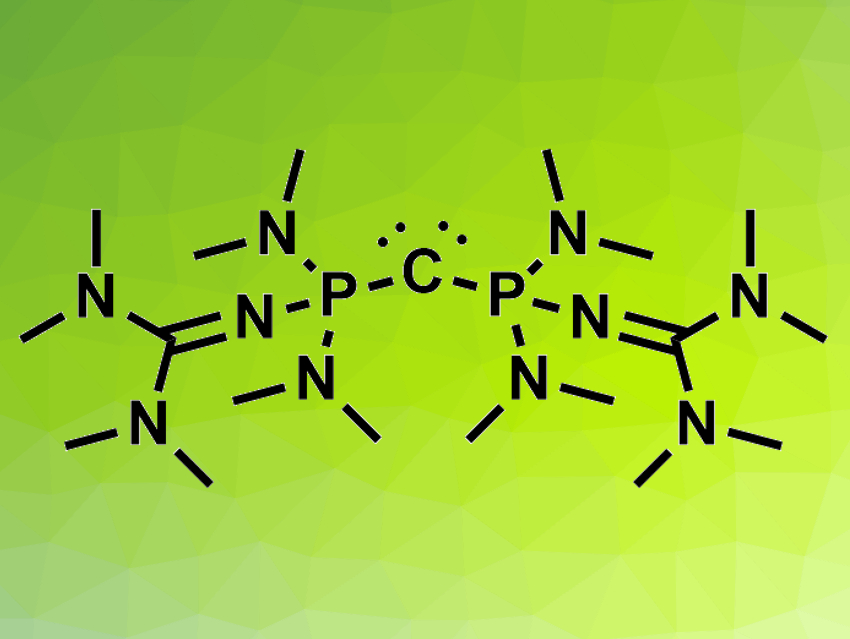
It is challenging to design non-ionic organic bases that match the basicity of inorganic bases. There are examples of highly basic nitrogen or phosphorus atoms in organic compounds. Non-ionic carbon can also act as a base: compounds such as carbodiphosphoranes (R3P−C−PR3) have two lone electron pairs that are mostly localized at the carbon atom. Using very electron-rich PR3 substituents could increase the already high basicity of the central carbon atom in these compounds.
Jörg Sundermeyer, University of Marburg, Germany, and colleagues have synthesized a new generation of superbasic carbodiphosphoranes. The team used electron-rich pyrrolidine, tetramethylguanidine, or hexamethylphosphazene as electron-rich substituents. They first prepared cationic precursors, which were deprotonated using bases such as potassium bis(trimethylsilyl)amide (KHMDS) or sodium amide (NaNH2).
The resulting carbodiphosphoranes’ basicity was determined using NMR titration experiments. The tetramethylguanidinyl-substituted product (pictured) is orders of magnitude more basic than the strongest uncharged carbon superbase known so far, H2C=P(2,4,6-(MeO)3-C6H2)2Ph. According to the researchers, the compounds could be useful in organic superbase catalysis.
- Design of Non-Ionic Carbon Superbases: Second Generation Carbodiphosphoranes,
Sebastian Ullrich, Borislav Kovačević, Björn Koch, Klaus Harms, Jörg Sundermeyer,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc03565f