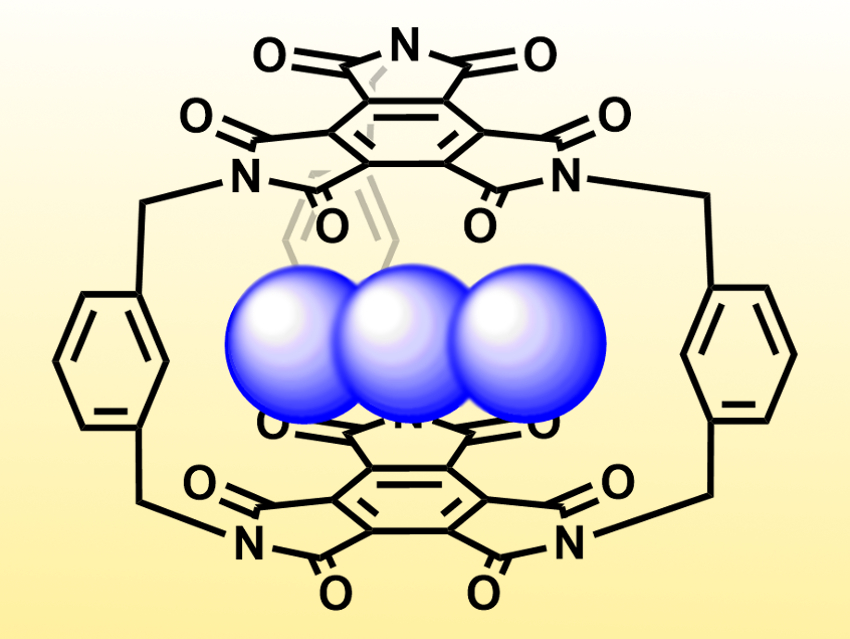
Benzene triimide (BTI) is a planar, electron-deficient, π-conjugated molecule. Due to its three imide groups, it is easy to functionalize and could be a useful building block for larger organic scaffolds.
Qi-Qiang Wang, De-Xian Wang, Beijing National Laboratory for Molecular Sciences, and University of the Chinese Academy of Sciences, Beijing, and colleagues have synthesized a BTI-based cage which can selectively bind anions. The team used three m-xylylene spacers to connect to molecules of BTI. This was achieved by reacting BTI with 1,3-bis(bromomethyl)benzene in the presence of N,N-diisopropylethylamine (DIPEA) as a base.
The resulting cage has an electron-deficient cavity which can bind anions. The team found that it can bind, e.g., N3– (azide, pictured), SCN–, and I–. Azide is bound particularly strongly and selectively—over 150 and 250 times more strongly than SCN– and I–, respectively. The team attributes this to the fact that azide is tightly trapped within the cavity and interacts strongly with the π-electron system.
- Benzene Triimide Cage as a Selective Container of Azide,
De-Hui Tuo, Yu-Fei Ao, Qi-Qiang Wang, De-Xian Wang,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b02782