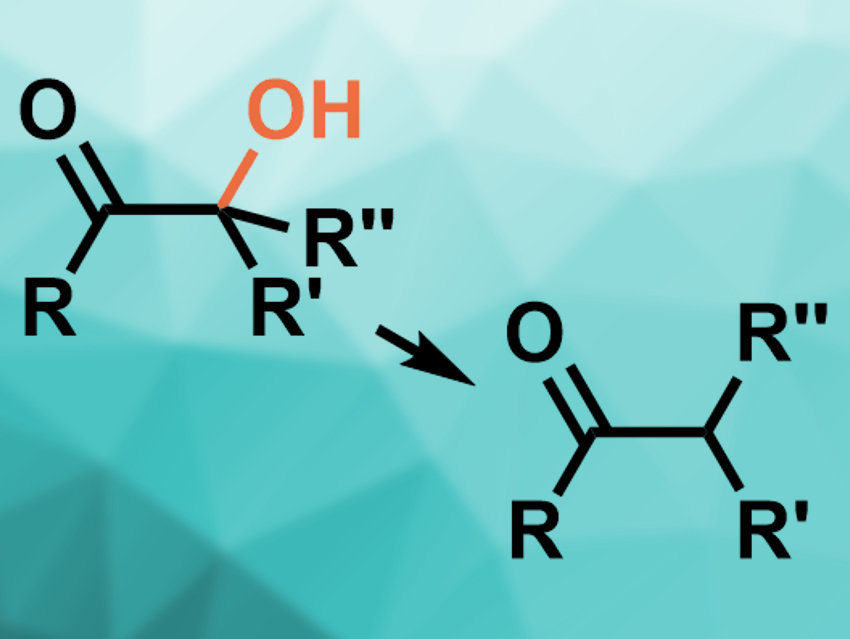
α-Ketols can be dehydrogenated to give ketones (pictured). Usually, metal-based reducing agents are used for this type of reaction (e.g., Zn, Li, or Sm reagents). Known approaches generally require dry conditions, multiple steps, and/or the protection of hydroxyl groups. They also do not provide good functional group tolerance.
Dmitry Tsvelikhovsky and colleagues, The Hebrew University of Jerusalem, Israel, have developed a metal-free, easy-to-operate, proline-promoted dehydroxylation of α-ketols. The team used proline as a reducing agent, potassium acetate as a base, and dimethylsulfoxide (DMSO) as the solvent. The reaction was performed at 100–130 °C and gives the desired ketones in generally good yields.
The reaction can be performed in an open flask and tolerates other functional groups. The method demonstrates a previously unknown reactivity of proline.
- Proline-Promoted Dehydroxylation of α-Ketols,
Dmitry Tsvelikhovsky, Yelena Mostinski, David Lankri, Yana Konovalov, Riva Nataf,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc02543j