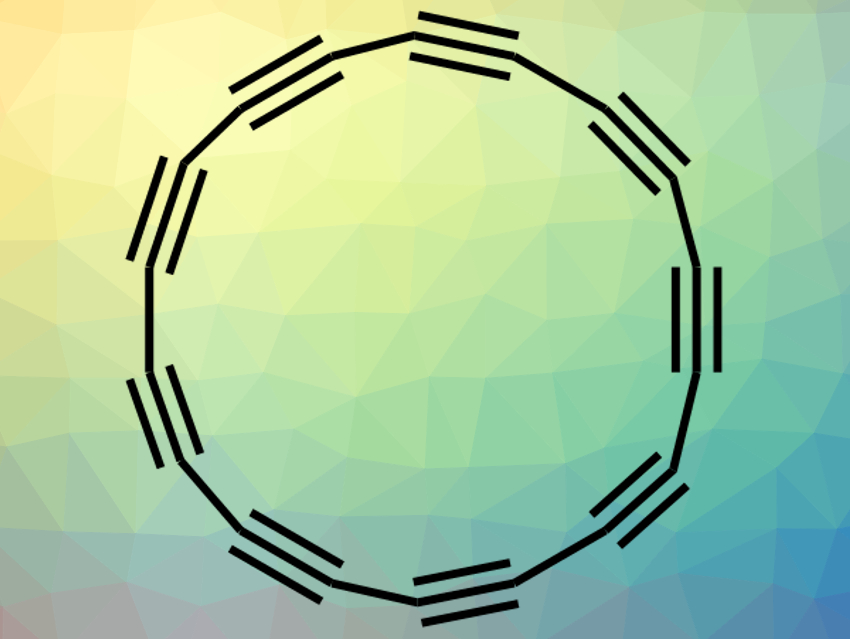
Carbon exists in many forms: diamonds, graphite, graphene, fullerenes, and carbon nanotubes are well-known allotropes. Rings of 2-coordinate, sp-hybridized carbon atoms have been suggested as another possible form. However, due to their high reactivity, such rings had not been isolated or characterized outside of the gas phase so far.
Przemyslaw Gawel, Harry L. Anderson, Oxford University, UK, Leo Gross, IBM Research–Zurich, Rüschlikon, Switzerland, and colleagues have synthesized such a ring: cyclo[18]carbon (C18, pictured). The team used low-temperature scanning tunneling microscopy and atomic force microscopy (STM–AFM) to prepare and observe the compound on an NaCl surface. They started from the cyclocarbon oxide C24O6 as a precursor and removed the six CO groups sequentially. This was achieved by manipulating the atoms using voltage pulses from the microscope tip. The researchers prepared C18 from C24O6 with a yield of 13 %.
C18 and its precursors can also be coupled on the surface using voltage pulses between to neighboring rings. Only a few examples of this type of reaction—on-surface covalent molecular fusion by atom manipulation—had been reported before. According to the team, the method could also be used to create other new carbon-rich molecules.
- An sp-hybridized molecular carbon allotrope, cyclo[18]carbon,
Katharina Kaiser, Lorel M. Scriven, Fabian Schulz, Przemyslaw Gawel, Leo Gross, Harry L. Anderson,
Science 2019.
https://doi.org/10.1126/science.aay1914