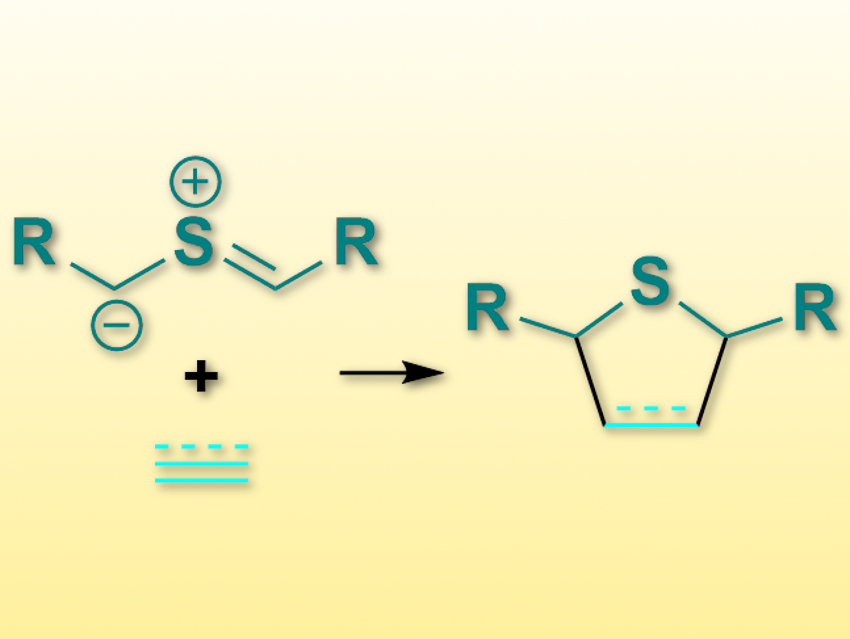
Cycloaddition reactions, e.g., 1,3-dipolar cycloadditions, can create a lot of chemical complexity in one step. One type of 1,3-dipolar compounds that have been rarely used so far are the thiocarbonyl ylides (pictured top left).
Thomas Magauer, University of Innsbruck, Austria, and colleagues have performed [3+2]-cycloadditions of thiocarbonyl ylides with a wide variety of alkenes and alkynes (pictured). Since the reaction volume of the cycloaddition is negative (i.e., the volume decreases), the team figured that high pressure could promote this reaction. They used bis(trimethylsilylmethyl) sulfoxides as the precursor for the desired thiocarbonyl ylides. This precursor was combined with alkenes or alkynes in N,N′-dimethylpropyleneurea (DMPU) at 23 °C and under 5–14 kbar pressure. The desired products, dihydro- or tetrahydrothiophenes (pictured right) were obtained in useful yields.
The team compared the high-pressure reaction to the thermal activation of the same reaction at 60–100 °C and found that the high-pressure variant had up to 58 % higher yields. It also allows the transformation of thermally unstable or sterically hindered substrates, which significantly broadens the substrate scope.
- A Synthetic Entry to Polyfunctionalized Molecules through the [3+2]-Cycloaddition of Thiocarbonyl Ylides,
Franz-Lucas Haut, Christoph Habiger, Klaus Speck, Klaus Wurst, Peter Mayer, Johannes Nepomuk Korber, Thomas Müller, Thomas Magauer,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b07729