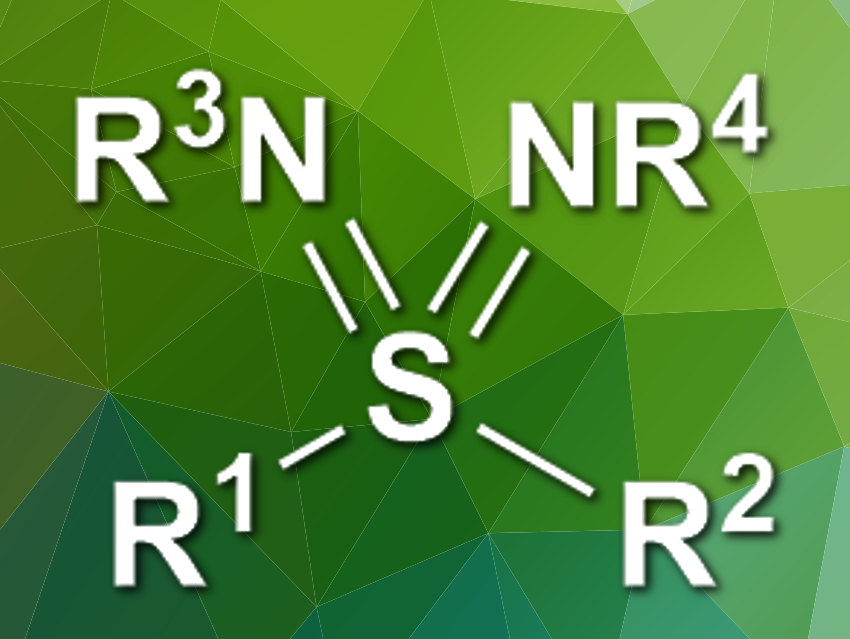
Sulfondiimines (pictured) are the aza-analogues of sulfones. They are promising, e.g., for pharmaceutical chemistry but can be challenging to synthesize. Existing methods start from smelly thiols and use hazardous reagents.
Michael C. Willis, University of Oxford, UK, and colleagues have developed an improved, modular synthesis of sulfondiimines. The team combined two Grignard reagents (R1–MgBr and R2-MgBr) with a sulfinylamine reagent (tOct–N=S=O, tOct = tert-octylamine) to give a sulfilimine intermediate (R1R2S=NtOct). A rhodium-catalyzed imination then gives doubly protected sulfondiimines with one tert-octyl and one nosyl protecting group. These can be deprotected individually to give NH groups that can be further functionalized.
This modular approach allowed the team to synthesize a wide variety of sulfondiimines. The sulfur center can be functionalized with alkyl, aryl, and heteroaryl groups. The intermediate sulfilimines can also be oxidized, which provides a path to sulfoximines.
- Modular Sulfondiimine Synthesis Using a Stable Sulfinylamine Reagent,
Ze-Xin Zhang, Thomas Q. Davies, Michael C. Willis,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b06831