Meroterpenoids are a type of natural product that are part terpene and part polyketide. They are chemically diverse and often biologically active.
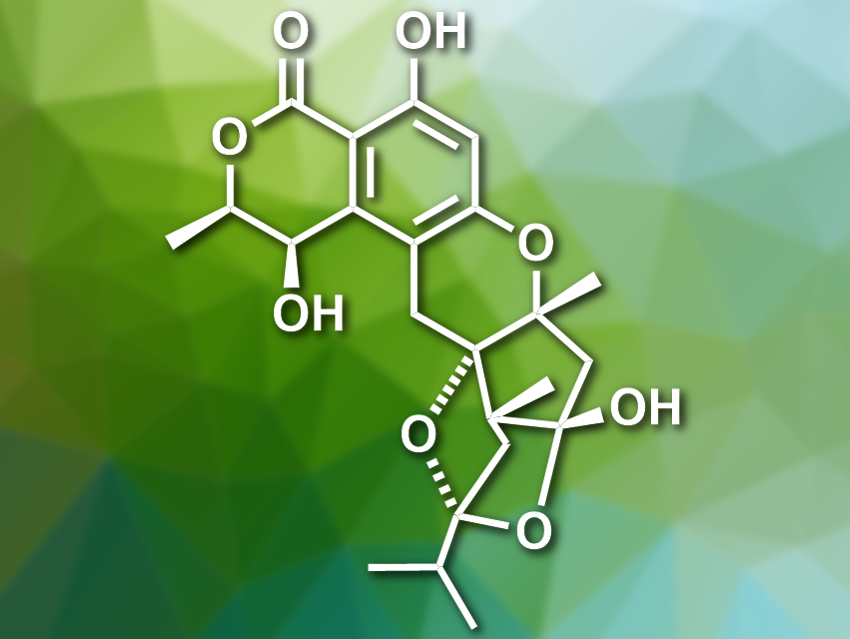
Bin Wu, Zhejiang University, Hangzhou, China, and colleagues have isolated five new meroterpenoids from a strain of Talaromyces marine fungi. The fungi were extracted with dichloromethane. The extract’s components were then separated using column chromatography and high-performance liquid chromatography (HPLC). The team obtained the meroterponoids talaromyolides A–D (A pictured) and talaromytin.
The structures of the new compounds were determined using NMR spectroscopy, mass spectrometry (MS), X-ray crystallography (XRC), and electronic circular dichroism (ECD) spectroscopy. Talaromytin, a heptacyclic product, was found to have two slowly interconverting conformers due to different orientations of its side chain. Talaromyolide A has a 6/6/6/5/5/5 polycyclic skeleton (pictured). Talaromyolide B and C both have a 6/6/6/6/6/6 hexacyclic skeleton and only differ by one substituent. Talaromyolide D contains one four-membered ring. It has potent antiviral activity against pseudorabies virus, a type of herpes virus in pigs. According to the researchers, this could make it an interesting research target.
- Talaromyolides A–D and Talaromytin: Polycyclic Meroterpenoids from the Fungus Talaromyces sp. CX11,
Xun Cao, Yutong Shi, Xiaodan Wu, Kuiwu Wang, Shaohua Huang, Hongxiang Sun, Jeroen S. Dickschat, Bin Wu,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b02466