Cycloparaphenylenes (CPPs) often have unique properties that are caused by their curved π-conjugated systems. They can, for example, be used as fluorophores. Cyclic molecules, in general, can have interesting reactivities caused by ring strain, whose release can be a driving force for reactions.
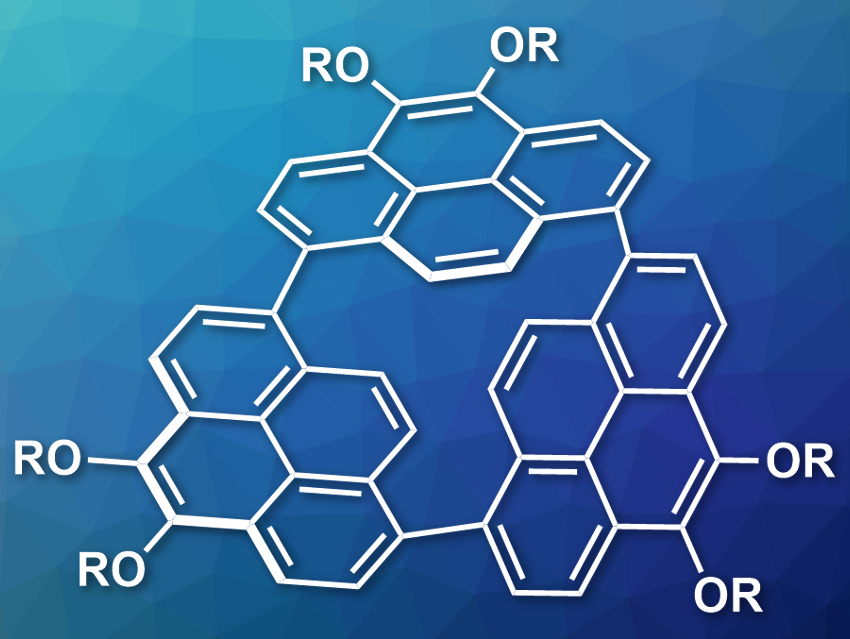
Naoki Aratani, Hiroko Yamada, Nara Institute of Science and Technology (NAIST), Japan, and colleagues have synthesized a strained, trimeric cycloparaphenylene (pictured, R = C3H7). The compound was prepared via a nickel-catalyzed coupling of 1,8-dibromo-4,5-dipropoxypyrenes. The trimer was separated from other products by chromatography.
The team found that the strained trimer has an unusual reactivity: Under ambient conditions and room light in air, the compound is converted into a biaryl ether at one of the Caryl–Caryl bonds. According to the researchers, this is the first metal-free direct oxygen atom insertion into a biaryl C–C σ-bond at ambient conditions. The color of emission changes from orange to light blue during this oxidation reaction. The reaction with O2 is driven by the release of ring strain.
- A remarkably strained cyclopyrenylene trimer that undergoes metal-free direct oxygen insertion into the biaryl C–C σ-bond,
Ryo Kurosaki, Hironobu Hayashi, Mitsuhara Suzuki, Julong Jiang, Miho Hatanaka, Naoki Aratani, Hiroko Yamada,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc01777a



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)