Carbon dioxide could be used as a renewable chemical feedstock. It can, for example, be reduced using H2 to give formic acid, dimethoxymethane, or methanol. In the preparation of formate from CO2, precious metal catalysts can achieve turn-over numbers (TONs) of up to several million. Non-precious metal catalysts, in contrast, usually only reach TONs of several thousand.
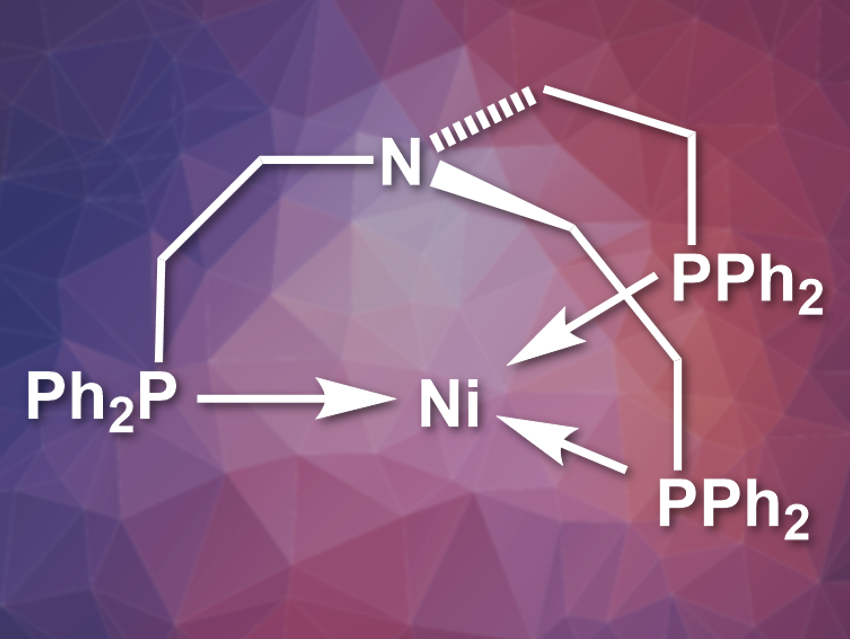
Jürgen Klankermayer and colleagues, RWTH Aachen University, Germany, have developed a nickel catalyst whose activity is comparable to precious-metal-based catalytic systems. The team used a multidentate ligand, tris(2-(diphenylphosphino)ethyl)amine), and a nickel(II) salt to prepare the catalyst (pictured). 1,8-Diazabicycloundec-7-ene (DBU) was used as a base. The reaction was performed under 30 bar CO2 pressure and a total pressure of 90 bar including H2.
At optimized reaction conditions, the catalyst achieved TONs of up to 4.65 · 106 in the homogeneous hydrogenation of CO2 to formate. The system, thus, outperforms other systems based on first-row transition metals. According to the researchers, detailed mechanistic investigations on the catalyst system are ongoing.
- A highly active non-precious transition metal catalyst for the hydrogenation of carbon dioxide to formates,
Benjamin G. Schieweck, Niklas F. Westhues, Jürgen Klankermayer,
Chem. Sci. 2019.
https://doi.org/10.1039/c8sc05230a