Redox-active organic molecules could be better than the commonly used inorganic materials for energy storage with high cycling stability. The organic skeleton of such molecules does not change much during reduction and oxidation. Inorganic compounds, in contrast, undergo large volume changes during charging and discharging, which can damage the material. However, most organic molecules do not conduct electricity and, thus, cannot be used as electrode materials.
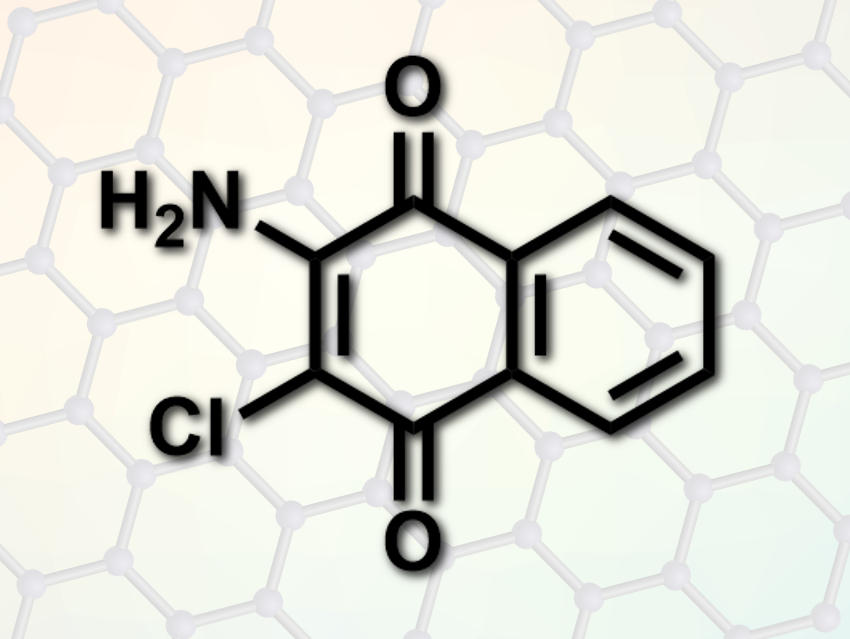
Zhongai Hu and colleagues, Northwest Normal University, Lanzhou, China, have bound redox-active organic molecules to electrically conductive graphene nanosheets to prepare an electrode material for energy storage. The team used 2-amino-3-chloro-1,4-naphthoquinone (ACNQ), which can exist in two redox forms. ACNQ was covalently bound to reduced graphene oxide via a diazotization reaction using isoamyl nitrite.
The resulting functionalized graphene nanosheets were used as one electrode in a supercapacitor to study their properties for energy storage. The researchers found that the supercapacitor has a high energy density of 19.1 Wh kg–1 at a power density of 0.8 kW kg–1. It also has a long cycling life, with almost no loss of capacity after 10,000 charge/discharge cycles.
- 2-Amino-3-chloro-1, 4-naphthoquinone-covalent modification of graphene nanosheets for efficient electrochemical energy storage,
Lijie Hou, Zhong-Ai Hu, Hongying Wu, Xiaotong Wang, Yandong Xie, Shanshan Li, Fuquan Ma, Cuimei Zhu,
Dalton Trans. 2019.
https://doi.org/10.1039/c9dt00895k