Supported metal nanoparticles (NPs) can act as catalysts for a variety of reactions. The properties of the support material, e.g., metal oxides or zeolites, can influence the catalysts’ reactivity.
Liang Wang, Zhejiang University, Hangzhou, China, Feng-Shou Xiao, Zhejiang University and Beijing University of Chemical Technology, China, Bruce C. Gates, University of California, Davis, USA, and colleagues have found that changing the support material can completely switch the reactivity of rhodium NP catalysts for carbon dioxide hydrogenation. The team prepared a zeolite with Rh NPs inside. They used NP-containing zeolite seeds for the crystallization of the material. The zeolites used were aluminosilicates with tunable Si/Al ratios. The researchers also synthesized a pure-silica variant of the zeolite with Rh NPs on the external surfaces using a controlled deposition method.
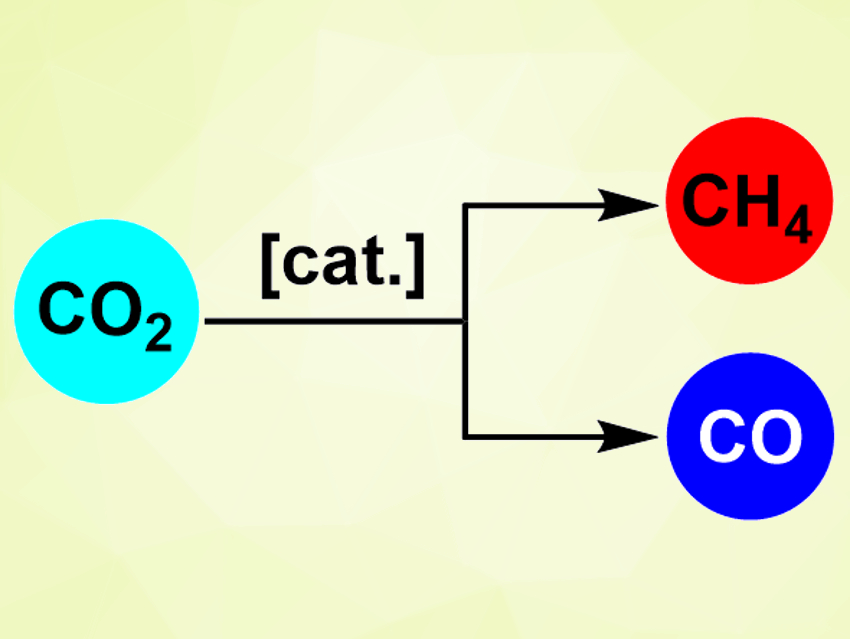
The team found that the Rh NPs inside the aluminosilicate zeolite selectively catalyze the conversion of CO2 to CH4 in the presence of H2 (methane selectivity of about 98 %). With Rh NPs on the pure silica support, the selectivity is changed significantly and CO is the predominant product (CO selectivity of about 80 %). The team attributes this difference to the support materials: the silica support favors the fast desorption of CO and limits hydrogenation, while the aluminosilicate zeolite favors the dissociation of hydrogen and, thus, the formation of CH4.
- Product Selectivity Controlled by Nanoporous Environments in Zeolite Crystals Enveloping Rhodium Nanoparticle Catalysts for CO2 Hydrogenation,
Chengtao Wang, Erjia Guan, Liang Wang, Xuefeng Chu, Zhiyi Wu, Jian Zhang, Zhiyuan Yang, Yiwen Jiang, Ling Zhang, Xiangju Meng, Bruce C. Gates, Feng-Shou Xiao,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b01555