Solid-state materials that contain hydrides have potential applications in, for example, hydrogen storage or catalysis. However, they are usually very reactive and sensitive to moisture. Finding a compromise between reactivity and stability, e.g., for metal borohydrides, is challenging. The reactivity of this type of compound depends on the metal ions and on hydrogen–hydrogen interactions in the crystal.
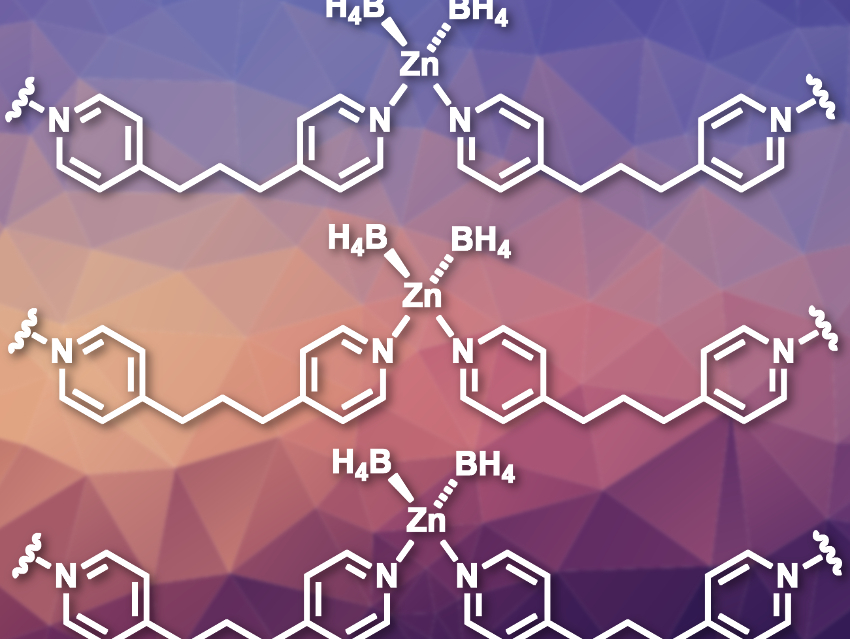
Satoshi Horike, Kyoto University, Japan, National Institute of Advanced Industrial Science and Technology (AIST), Kyoto, Japan, and Vidyasirimedhi Institute of Science and Technology, Rayong, Thailand, and colleagues have developed coordination polymers containing borohydride ions (BH4–). In these materials, the reactivity of the BH4– is regulated by the neighboring ligands. The team prepared crystals of the polymeric [Zn(BH4)2(dipyridylpropane)] (pictured) by layering solutions of [PPh4Zn(BH4)3] and dipyridylpropane.
The resulting [Zn(BH4)2(dipyridylpropane)] is composed of tetrahedrally coordinated Zn units connected by the dipyridine ligands to form chains. The polymer is stable in air with 50 % relative humidity, but still shows high reactivity for dehydrogenation. This is due to the crystal structure: The negatively charged hydrides interact with positively charged hydrogen atoms in the ligands, which stabilizes the material without affecting the reactivity too much. This type of interaction is called dihydrogen bonding.
- Borohydride-containing coordination polymers: synthesis, air stability and dehydrogenation,
Kentaro Kadota, Nghia Tuan Duong, Yusuke Nishiyama, Easan Sivaniah, Susumu Kitagawa, Satoshi Horike,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc00731h