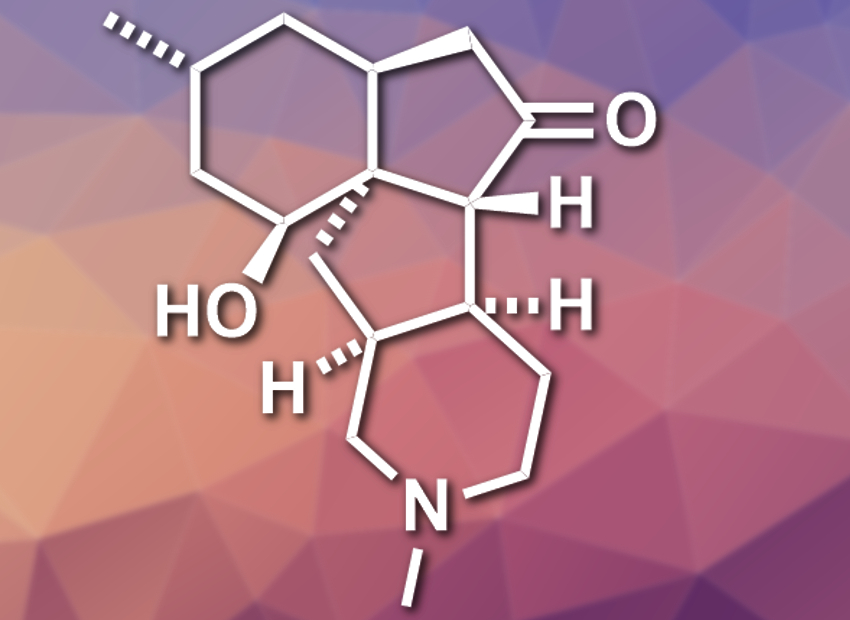
Lycopodium alkaloids are a class of natural products with high structural diversity and biological activities. Lycopodium is a genus of clubmosses from which these compounds were isolated. (+)-Paniculatine (pictured) from Lycopodium paniculatum has a tetracyclic framework with seven stereogenic centers. While there are several total syntheses for (+)-paniculatine and similar alkaloids, they involve between 25 and 45 steps.
Fayang G. Qiu, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, and University of the Chinese Academy of Sciences, Beijing, and colleagues have developed a concise total synthesis of (+)-paniculatine. The team started from two known intermediates: a thioether-substituted cyclohexanone and an iodine-substituted cyclic enone. These two compounds were coupled, allylated, and converted to a iodohydrin. Then the third ring was closed using a SN2 cyclization. The tetracyclic system was completed using an intramolecular Michael addition.
The synthesis has ten steps in total and gives an overall yield of 12 %. According to the researchers, the same strategy could be used for the synthesis of two related natural products, (−)-magellanine and (+)-magellaninone.
- A Concise Total Synthesis of (+)‐Paniculatine,
Fayang Qiu, Jian Liu, Sheng Chen, Ningning Li,
Adv. Synth. Catal. 2019.
https://doi.org/10.1002/adsc.201900376