Nitriles, or cyano compounds, are versatile intermediates in organic synthesis. Radical chemistry can be used to install cyano groups. There are methods for radical cyanations and cyanoethylations. Radical cyanomethylations, in contrast, had not been known so far.
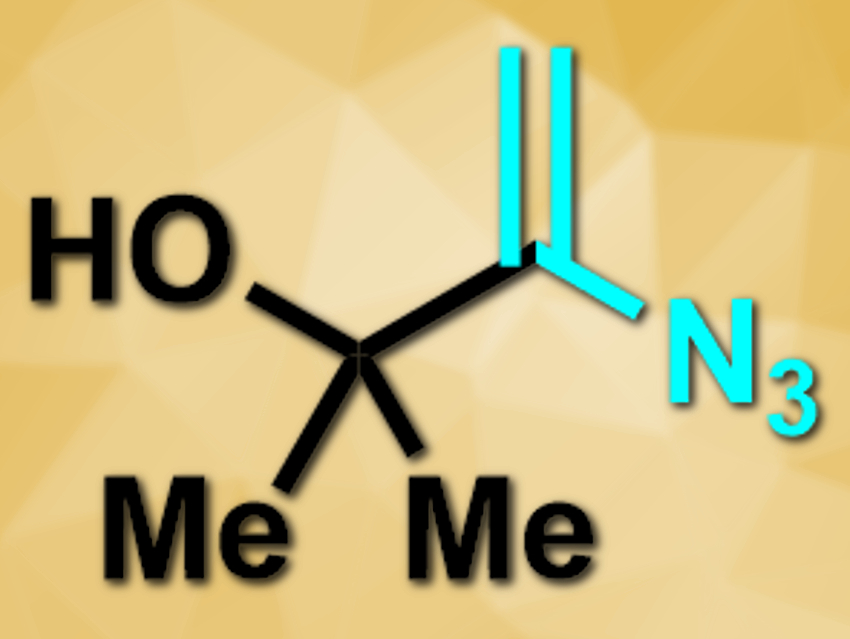
James R. Donald and Sophie L. Berrell, University of York, UK, have developed a method for radical cyanomethylation reactions. The team used the vinyl azide reagent 3-azido-2-methylbut-3-en-2-ol (pictured), which can take part in radical additions that trigger a cascade-fragmentation mechanism. The vinyl azide reagent loses dinitrogen and the stabilized 2-hydroxypropyl radical and, thus, acts as a cyanomethyl equivalent.
The reagent was combined with a photocatalyst, e.g., Ru(bpy)3Cl2·6H2O or fac-Ir(ppy)3 (bpy = 2,2′-bipyridine, ppy = 2-phenylpyridine), 2,6-lutidine, and a variety of precursors for α-carbonyl-, heterobenzylic, alkyl-, sulfonyl-, or aryl radicals in acetonitrile under blue LED light. The vinyl azide reagent traps the resulting radicals and the desired cyanomethylated products are obtained in moderate to excellent yields. According to the researchers, the reaction’s mild conditions allow it to be used, e.g., in the late-stage cyanomethylation of pharmaceutical agents.
- Radical cyanomethylation via vinyl azide cascade-fragmentation,
James R Donald, Sophie L. Berrell,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc01370a