Olefin metathesis is a commonly used method to create new C=C bonds, e.g., in ring-closing reactions or polymerizations. These reactions are usually catalyzed by transition metal complexes. Finding methods to switch these reactions on or off on demand could be useful. This can be achieved, for example, using heat, light, or pH changes. However, the control of metathesis reactions with light has required UV radiation so far.
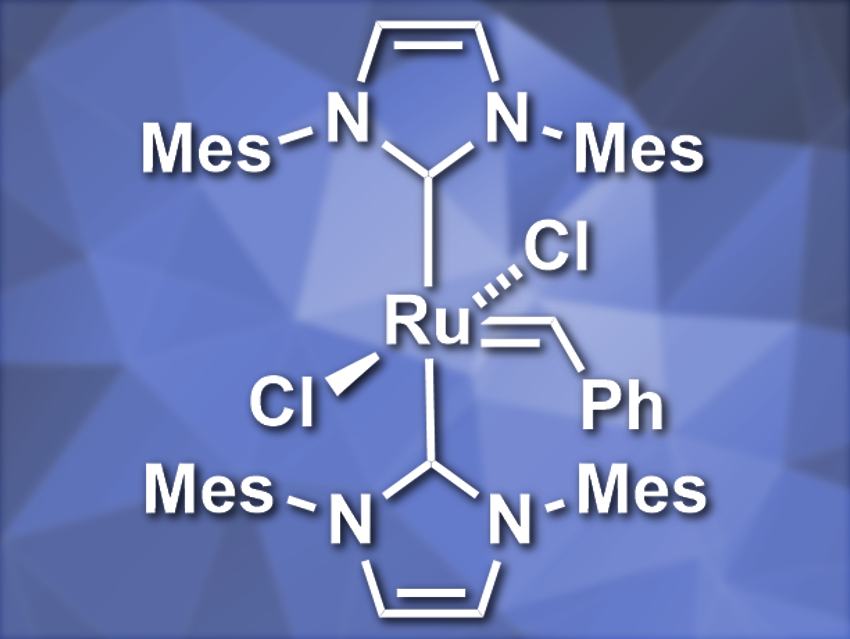
Tomislav Rovis and colleagues, Columbia University, New York, USA, have developed a system for visible-light-controlled olefin metathesis that combines a ruthenium pre-catalyst (pictured, Mes = 2,4,6-trimethylphenyl) with photoredox catalysis. The ruthenium precatalyst has two N-heterocyclic carbene (NHC) ligands and is inactive under ambient conditions. The team combined it with 2,4,6-triphenylpyrylium tetrafluoroborate (TPPT) as a photocatalyst. Under visible-light irradiation using blue LEDs, the photocatalyst promotes the oxidation of the precatalyst. This leads to the dissociation of one NHC ligand and activates the catalyst for olefin metathesis.
The team used this catalyst system to promote a range of light-activated cross metathesis and ring-closing metathesis reactions and obtained the desired products in good to excellent yields. The system was also successfully used for ring-opening metathesis polymerizations (ROMPs). This could enable applications, e.g., in photolithography or 3D printing.
- Visible-Light-Controlled Ruthenium-Catalyzed Olefin Metathesis,
Cédric Theunissen, Melissa A. Ashley, Tomislav Rovis,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.8b13663