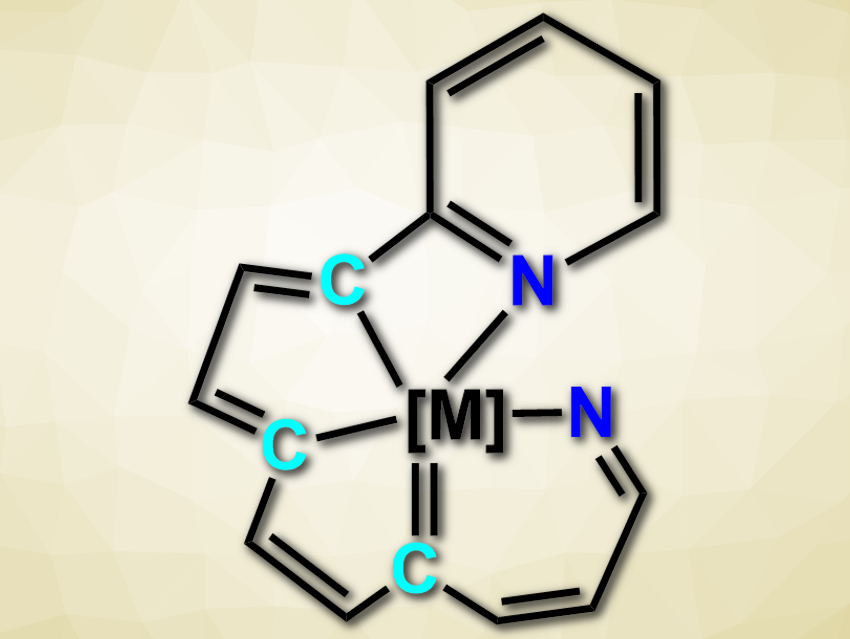
Chelate ligands, i.e., ligands with multiple bonding atoms, are very common in nature and useful in synthetic chemistry. Planar ligands with both carbon and nitrogen as coordinating atoms, for example, are used in a variety of applications. While tetradentate ligands (i.e., ligands with four coordinating atoms) of this type are well known, pentadentate or higher binding C/N ligands can be difficult to access.
Hong Zhang, Xiamen University, China, and colleagues have developed an efficient synthesis of tetradentate CCCN and pentadentate CCCCN and NCCCN ligands (example pictured). The team started from a tetradentate ligand with a CCCC core in the form of an osmium complex and successively modified this substrate to give the desired products.
The team first reacted the precursor with 2-ethynylpyridine to give a pentadentate CCCCN-type complex. Treating the product with azidotrimethylsilane and tetrabutylammonium fluoride causes the replacement of one carbon atom with a nitrogen atom and gives an NCCC-type complex. Aromatization of this complex, followed by protonation, leads to a pentadentate NCCCN-type complex. According to the researchers, the developed reactions provide a rare approach to mixed, high-coordinate, planar C/N ligands.
- Successive modification of polydentate complexes gives access to planar carbon- and nitrogen-based ligands,
Xiaoxi Zhou, Xin Pang, Liming Nie, Congqing Zhu, Kaiyue Zhuo, Qingde Zhuo, Zhixin Chen, Gang Liu, Hong Zhang, Zhenyang Lin, Haiping Xia,
Nat. Commun. 2019.
https://doi.org/10.1038/s41467-019-09367-8