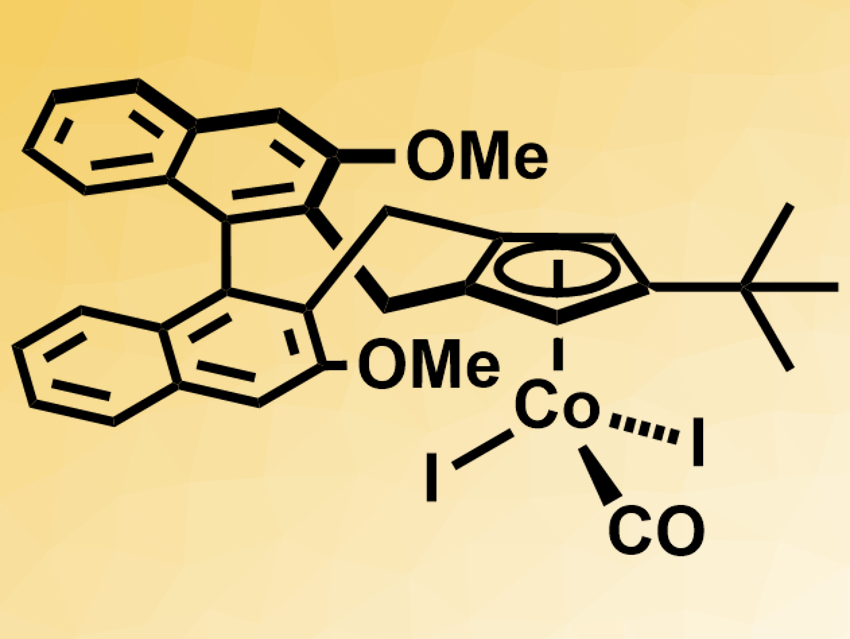
Enantioselective C–H functionalizations usually require precious-metal catalysts with chiral ligands. Using the more abundant 3d transition metals could make such reactions more cost-effective and sustainable. However, this is still challenging.
Nicolai Cramer and colleagues, École Polytechnique Fédérale de Lausanne (EPFL), Switzerland, have developed a cobalt(III)-based catalyst with a chiral cyclopentadienyl-type ligand (pictured). The catalyst can promote enantioselective C–H functionalizations. The team prepared the complex from the cyclopentadienyl ligand and Co2(CO)8 under a CO atmosphere, followed by an oxidation using iodine.
The catalyst was used for the enantioselective synthesis of dihydroisoquinolones from N-chlorobenzamides and different alkenes. The team used a catalyst loading of 5 mol%, together with silver triflate as an additive and cesium pivalate as a base in hexafluoroisopropanol (HFIP) as a solvent. The desired products were obtained in good yields and with excellent enantio- and regioselectivities (up to > 99:1 er).
- Chiral Cyclopentadienyl Cobalt(III) Complexes Enable Highly Enantioselective 3d-Metal-Catalyzed C-H Functionalizations,
Kristers Ozols, Yun-Suk Jang, Nicolai Cramer,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b02569