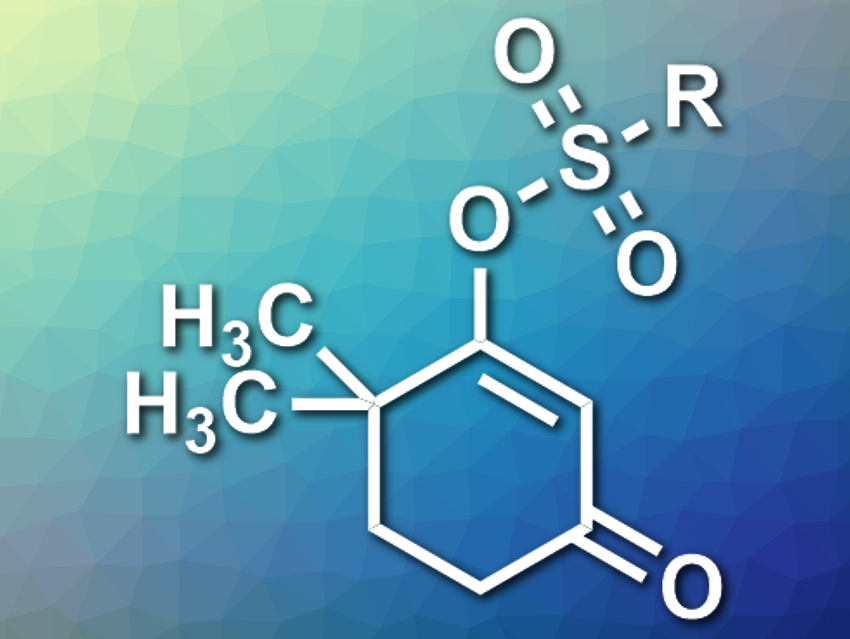
Vinylogous acyl sulfonates, i.e., sulfonates that are separated from an acyl group by one conjugated C=C bond (example pictured) are useful intermediates in synthetic organic chemistry. They are usually synthesized from 1,3-dicarbonyl compounds using a sulfonate electrophile. However, this reaction gives the products as a mixture of two isomers when the substrate is unsymmetrically substituted.
James K. Tucker and Matthew D. Shair, Harvard University, Cambridge, MA, USA, have developed a regioselective synthesis of vinylogous acyl sulfonates. The team used the corresponding vinyl sulfonates as substrates and performed an allylic oxidation to create the acyl functionality. FeCl3 was used as a catalyst, tert-butyl hydroperoxide (TBHP) as an oxidant, and a mixture of water and acetonitrile as the solvent.
The reaction has a high regioselectivity and gives the desired vinylogous acyl sulfonates in moderate to good yields. The method performs best when there is a quarternary center next to the sulfonate group. The products can be subjected to a variety of further chemical transformations.
- Catalytic Allylic Oxidation to Generate Vinylogous Acyl Sulfonates from Vinyl Sulfonates,
James K. Tucker, Matthew D. Shair,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b00845