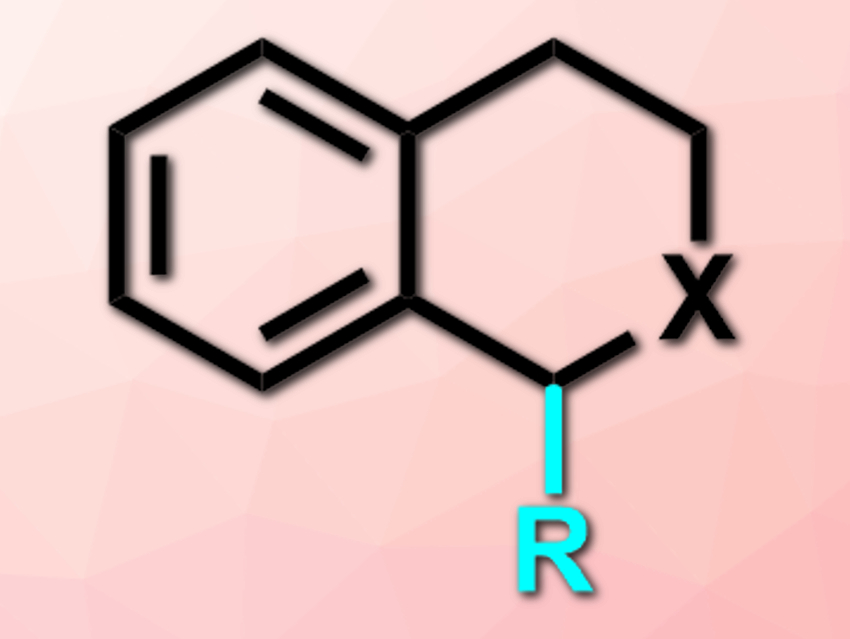
Zhihua Peng and colleagues, China University of Petroleum (East China), Qingdao, have developed an efficient C(sp3)–H bond alkylation of tetrahydroisoquinolines (THIQs) or isochroman with alkylzinc reagents (product pictured; X = NR’ or O; R = alkyl). THIQs and isochroman derivatives show a variety of biological activities, which makes these compounds interesting for pharmaceutical chemistry.
The team used phenyliodine bis(trifluoroacetate) (PIFA) as an oxidant to convert THIQs (X = NR’) to iminium cations in dichloroethane (DCE) at 80 °C. These were then reacted with alkylzinc reagents coordinated with MgCl2 and LiCl at 25 °C to give the desired products. The less reactive isochroman (X = O) can be converted under the same conditions.
The reaction proceeds without a heavy-metal catalyst under mild conditions. It tolerates sensitive functional groups such as cyano groups or boronic acid pinacol esters and can be performed on a gram scale.
- Direct and Efficient C(sp3)–H Bond Alkylation of Tetrahydroisoquinolines and Isochroman with Alkylzinc Reagents,
Zhihua Peng, Yilei Wang, Zhi Yu, Hao Wu, Shanshan Fu, Linhua Song, Cuiyu Jiang,
Adv. Synth. Catal. 2019.
https://doi.org/10.1002/adsc.201900023