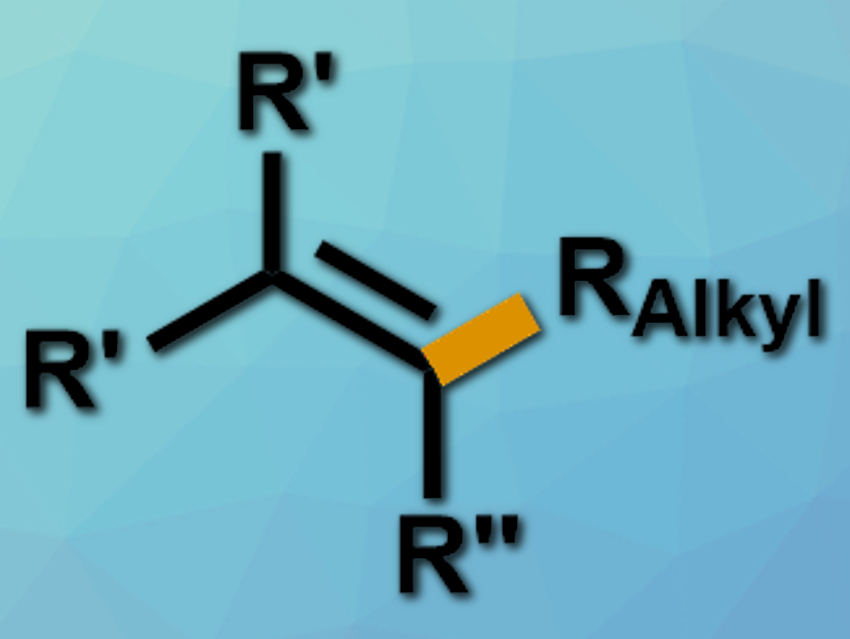
Cross-coupling reactions can form C–C bonds. Palladium-catalyzed cross-coupling reactions of alkenyl halides with organolithium compounds, for example, have been known for a long time. However, palladium catalysts are expensive. Catalysts based on earth-abundant metals such as iron would be a low-cost and environmentally friendly alternative.
Xiao-Shui Peng, Henry N. C. Wong, The Chinese University of Hong Kong, Shatin, and colleagues have developed a protocol for a stereoselective iron-catalyzed cross-coupling between alkyllithium compounds and alkenyl iodides (example product pictured). The team used Fe(acac)2 as the catalyst (acac = acetylacetonate), DavePhos (2-dicyclohexylphosphino-2′-(N,N-dimethylamino)biphenyl) as a ligand, and toulene as a solvent to couple different alkyllithium compounds with a variety of alkenyl iodides at 23 °C.
The desired products were obtained in moderate to good yields and with high stereospecificity. The reaction can be performed on a gram scale. The unsaturated products are useful for further chemical transformations.
- Stereospecific Iron-Catalyzed Carbon(sp2)–Carbon(sp3) Cross-Coupling with Alkyllithium and Alkenyl Iodides,
Xiao-Lin Lu, Mark Shannon, Xiao-Shui Peng, Henry N. C. Wong,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b00394